Published online Mar 20, 2021. doi: 10.5493/wjem.v11.i2.17
Peer-review started: December 24, 2020
First decision: January 18, 2021
Revised: February 19, 2021
Accepted: March 12, 2021
Article in press: March 12, 2021
Published online: March 20, 2021
Hypoxic-ischemic encephalopathy (HIE) is a leading cause of morbidity and mortality in the adult as well as in the neonate, with limited options for treatment and significant dysfunctionality.
To investigate the safety and preliminary efficacy of allogeneic mesenchymal stem cells (MSCs) in HIE patients.
Patients who had HIE for at least 6 mo along with significant dysfunction and disability were included. All patients were given Wharton’s jelly-derived MSCs at 1 × 106/kg intrathecally, intravenously, and intramuscularly twice a month for two months. The therapeutic effects and prognostic implications of MSCs were evaluated by multiple follow-ups. Functional independence measure (FIM), modified Ashworth, and Karnofsky scales were used to assess any side effects, neurological and cognitive functions, and overall outcomes.
The 8 subjects included in the study had a mean age of 33.25 ± 10.18 years. Mean HIE exposure and mean post-HIE durations were 45.63 ± 10.18 and 19.67 ± 29.04 mo, respectively. Mean FIM score was 18.38 ± 1.06, mean modified Ashworth score was 43.5 ± 4.63, and mean Karnofsky score was 20. For the first 24 h, 5 of the patients experienced a subfebrile state, accompanied by mild headaches due to intrathecally administration and muscle pain because of intramuscularly administration. Neurological and functional examinations, laboratory tests, electroencephalography, and magnetic resonance imaging were performed to assess safety of treatment. Mean FIM score increased by 20.88 ± 3.31 in the first month (P = 0.027) and by 31.38 ± 14.69 in 12 mo (P = 0.012). The rate of patients with an FIM score of 126 increased from 14.58% to 16.57% in the first month and 24.90% in 12 mo.
Multiple triple-route Wharton’s jelly-derived MSC administrations were found to be safe for HIE patients, indicating neurological and functional improvement. Based on the findings obtained here, further randomized and placebo research could be performed.
Core Tip: Occurring due to the disruption of oxygen supply to the brain, hypoxic-ischemic encephalopathy is associated with substantial rates of morbidity and mortality. In addition to supportive therapy and symptomatic treatment, research on the treatment of hypoxic-ischemic encephalopathy has focused new therapautic strategies as stem cell therapy. This multi-center and open-label phase I study was performed to investigate the safety and preliminary efficacy of multiple triple-route Wharton’s Jelly-Derived Mesenchymal Stem Cells administrations. The patients included in this study also had improvement in modified Ashworth scores, Functional Independence Measure scores over the course of a year, indicating long-term impact on brain functions.
- Citation: Kabataş S, Civelek E, Kaplan N, Savrunlu EC, Sezen GB, Chasan M, Can H, Genç A, Akyuva Y, Boyalı O, Diren F, Karaoz E. Phase I study on the safety and preliminary efficacy of allogeneic mesenchymal stem cells in hypoxic-ischemic encephalopathy. World J Exp Med 2021; 11(2): 17-29
- URL: https://www.wjgnet.com/2220-315x/full/v11/i2/17.htm
- DOI: https://dx.doi.org/10.5493/wjem.v11.i2.17
Occurring due to the disruption of oxygen supply to the brain, hypoxic-ischemic encephalopathy (HIE) is associated with substantial rates of morbidity and mortality in both infants and adult patients[1,2]. Inflammation and neurovascular damage constitute potential warning factors for therapeutic intervention[3]. However, there are inadequate standards and specific measures available for the treatment of HIE. Recently, in addition to supportive therapy and symptomatic treatment, research on the treatment of HIE has focused on the following aspects: hypothermia therapy, neuroprotective agents, etc[4]. Another new therapeutic tool with promising indications for clinical practice is stem cell therapy[5]. There are ever-increasing evidences on mesenchymal stem cells (MSCs), suggesting that they promote the improvement of neurological functions, with significant immunomodulatory effects against neuroinflammatory events[6,7]. Bone marrow-derived MSCs (BM-MSCs) and umbilical cord blood-derived MSCs are now being included in efforts to prevent ischemic brain damage[8]. Although they constitute a major source of MSCs, BM-MSCs are seldom preferred due to the high incidence of viral infection and the low number of cells[9]. On the other hand, fetal-derived MSCs, which are more primitive and have less immune reactivity, have recently been suggested as better alternatives for BM-MSCs. Thus, Wharton’s jelly-derived MSCs (WJ-MSCs), can easily be obtained in abundance and are readily cultured, making them good alternatives[10].
The optimal route of MSC administration remains a question of significance. While efficient and useful for avoiding negative outcomes often encountered in invasive procedures, intravenous (IV) application alone may lead to retainment in other organs, including the liver, the spleen, the kidneys, and the lungs[11]. Multiple-route administration could therefore be a better alternative, with research supporting no side effects by IV and intrathecal (IT) administration[10]. We previously reported multiple triple-route WJ-MSC administrations to be safe and applicable for HIE and cerebral palsy patients[12,13]. Based on the further research on the matter, WJ-MSCs can now be used for the clinical treatment of HIE.
This phase I study aimed to investigate the effects and preliminary estimates of multiple triple-route WJ-MSC administrations. The population of the study consisted of HIE patients with significant dysfunction. Primary outcome was considered safety of application by neurological and functional examinations, laboratory tests, electroencephalography, and magnetic resonance imaging.
This multi-center and open-label phase I study was performed to investigate the safety and preliminary efficacy of multiple triple-route WJ-MSC administrations. Inclusion criteria were being an adult and having HIE and significant impairment and dysfunctionality (Table 1). The study made no restrictions on, and did not provide any forms of, medication or therapy (occupational, physical, or speech) during the follow-up year after WJ-MSC applications. Written informed consent forms were obtained from the legal representatives of the patients. Approval for the trial was obtained from the Turkish Ministry of Health, General Directorate of Health Services, Department of Organ/Tissue Transplantation and Dialysis Services, and the Scientific Committee (No. 56733164-203-E.2569). The findings are given in detail in Table 2.
| Inclusion criteria |
| Age ≥ 18 |
| HIE ≥ 6 mo prior, radiologically confirmed at initial diagnosis and at study enrollment |
| The patients who does not have any chronic illness (cancer, kidney, heart/hepatic failure etc.) other than HIE. Adequate systemic organ function confirmed by normal ranged laboratory values |
| Life expectancy > 12 mo |
| No substiantial improvement despite of a treatment in neurological/functional status for the 3 mo before study enrollment |
| Severe disability defined as subject confined to a wheelchair/required to have home nursing care/needing assistance with activities of daily living |
| Expectation that the patient will receive standard post-treatment care and attend all visits |
| Signing in the written informed consent form for confirming to that know the treatment to be applied and to be willing by their parents/a surrogate |
| Exclusion criteria |
| Presence of any other clinically significant medical/psychiatric condition, or laboratory abnormality, for which study participation would pose a safety risk in the judgment of the investigator/sponsor or history within the past year of drug/alcohol abuse |
| Recently diagnosed severe infection (meningitis, etc.)/development of liver, kidney/heart failure/sepsis or skin infection at the i.v. infusion site or positive for Hepatitis B, C/HIV |
| History of uncontrolled seizure disorder |
| History of cerebral neoplasm, or cancer within the past 5 yr, with the exception of localized basal or squamous cell carcinoma |
| Having clinic symptoms that formation of white sphere number ≥ 15000/µL or platelet count ≤ 100.000/µL |
| Serum aspartate aminotransferase and serum alanine aminotransferase > 3 × upper limit of normal/creatinine > 1.5 × upper limit of normal |
| Pregnant/lactating/expectation to become pregnant during the study |
| Participation in an another investigational stem cell study before treatment |
| The patient/parents decides to abandon the treatment or the patient death |
| Frequency | Percent | ||
| Age | 20.00 | 1 | 11.1 |
| 25.00 | 1 | 11.1 | |
| 27.00 | 1 | 11.1 | |
| 29.00 | 1 | 11.1 | |
| 34.00 | 1 | 11.1 | |
| 37.00 | 1 | 11.1 | |
| 43.00 | 1 | 11.1 | |
| 51.00 | 1 | 11.1 | |
| Sex | M | 8 | 100.0 |
| F | 0 | 0 | |
| Cause of hypoxia | Cardiac arrest | 1 | 12.5 |
| Cardiac arrest due to acute myocard infarction | 3 | 37.5 | |
| Cardiac arrest due to explosive devices injury | 1 | 12.5 | |
| Cardiac arrest due to multi-trauma | 1 | 12.5 | |
| Cardiac arrest, unkown ethiology | 2 | 25.0 | |
| Duration of hypoxia | 25.00 | 1 | 12.5 |
| 30.00 | 1 | 12.5 | |
| 40.00 | 1 | 12.5 | |
| 45.00 | 3 | 37.5 | |
| 60.00 | 1 | 12.5 | |
| 75.00 | 1 | 12.5 | |
| Previous treatment | No | 8 | 100.0 |
| Yes | 0 | 0 | |
| Comorbidity | Atrial fibrilation | 1 | 12.5 |
| No | 7 | 87.5 | |
| Duration between hypoxia and first SCT | 6.00 | 3 | 37.5 |
| 10.00 | 1 | 12.5 | |
| 11.00 | 1 | 12.5 | |
| 18.00 | 2 | 25.0 | |
| 96.00 | 1 | 12.5 | |
Umbilical cords were obtained from various donors at the Good Manufacturing Practice facility of LivMedCell (Istanbul, Turkey), with informed consents approved by the institutional regulatory board. Postnatal umbilical cords were obtained from donors with full-term pregnancy[12,13].
Umbilical cords were washed with phosphate-buffered saline (Invitrogen/Gibco, Paisley, United Kingdom). After removing the blood vessels, tissues were cut into explants of 5 to 10 mm3 and cultured in humanized culture conditions (5% CO2 at 37 °C) until cell migration occurred. The cells were harvested once they reached 70%-80% confluency and characterization tests were conducted at passage 3. Quality control was done in accordance with the standards of the Turkish Agency of Medicines and Medical Devices[12,13].
According to flow cytometry results, the stem cells were positive for CD44, CD73, CD90, and CD105 and negative for CD34, CD45, and HLA-DR. Their telomerase activities were found to be stable throughout culturing, with a large and flattened morphology[12,13].
Expression of some markers was found, including TERT, POU5F1, SOX2, ZFP42, CD44, VCAM1, THY1, BMP2, RUNX-1, ICAM1, and NES. Differentiation tests showed that the cells had a capacity for trilineage. Although, karyotyping did not yield any structural or chromosomal abnormality[12,13].
The final WJ-MSC preparations were harvested from cell culture passage 3 and suspended in densities of 1 × 106 in 3, 20, and 30 mL of normal saline[12,13].
All patients underwent examination in anesthesia and reanimation, neurology, physical therapy and rehabilitation, and neurosurgery departments. After ensuring that there is no contraindication for anesthesia, and no serious infectious disease, transplantation was carried out[12]. All procedures were performed by one team of doctors, including IT, IV, and intramuscular (IM) administration of allogeneic WJ-MSCs, under Sedo-anesthesia (Table 3). IT administration was done through lumbar puncture[14]. IM administration was performed under the guidance of ultrasonography. IV administration was done slowly over a period of 30 min. Then, the patients were sent to the intensive care unit for follow-up. Physical therapy and rehabilitation were initiated on the next day, avoiding exercise on the days of administration.
| Rounds | Route | WJ-MSC |
| Round 1 | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL | |
| Round 2 (2nd week) | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL | |
| Round 3 (4th week) | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL | |
| Round 4 (6th week) | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL |
Prior to treatment, all patients underwent immense neurological and functional assessment. Modified Ashworth (MA) scale was used to evaluate for spasticity and Functional independence measure (FIM) scale was used to evaluate for quality of life[15].
The criteria for safety of administration were the lack of infection, fever, headache, pain, increased C-reactive protein levels or leukocytosis, allergic reaction or shock, and complications associated with anesthesia or analgesia for 7 to 14 d. The criteria for safety of WJ-MSC were the lack of infection, neuropathic pain, cancer, and neurological deterioration for 1 year[12,13].
Besides MA and FIM, Karnofsky scale was used to evaluate the outcome[12,13]. Assessments also included investigation for neuropathic pain, secondary infections, urinary tract infections, or pressure ulcers of the skin.
Of nonparametric tests, Friedman and Wilcoxon Signed Rank tests were used to measure changes in FIM, MA, and Karnofsky scale scores before and after the operation. The reason for using nonparametric tests was the inadequate number of data for parametric tests.
The patients showed good tolerance, with no severe side effects associated with the administration. For the first 24 h, 5 of the patients experienced a subfebrile state, accompanied by mild headaches due to IT administration and muscle pain because of IM administration (Table 4). There were no problems of safety or side effects during the 1-year follow-up.
| Complications | Patient No: 1 | Patient No: 2 | Patient No: 3 | Patient No: 4 | Patient No: 5 | Patient No: 6 | Patient No: 7 | Patient No: 8 | |||||||||||||||||||||||||
| Administration | Administration | Administration | Administration | Administration | Administration | Administration | Administration | ||||||||||||||||||||||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | ||
| Early | Infection | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fever | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | + | + | - | - | - | + | - | - | - | - | - | - | + | + | - | - | |
| Pain | - | - | - | - | - | - | - | - | + | - | + | - | + | + | - | - | + | - | - | - | - | + | - | - | - | - | - | - | + | - | + | - | |
| Headache | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | + | + | - | - | |
| Increased level of C-reactive protein | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Leukocytosis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Allergic reaction or shock | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Perioperative complications | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Late | Secondary infections | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Urinary tract infections | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Deterioration of neurological status | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Neuropathic pain | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Carcinogenesis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
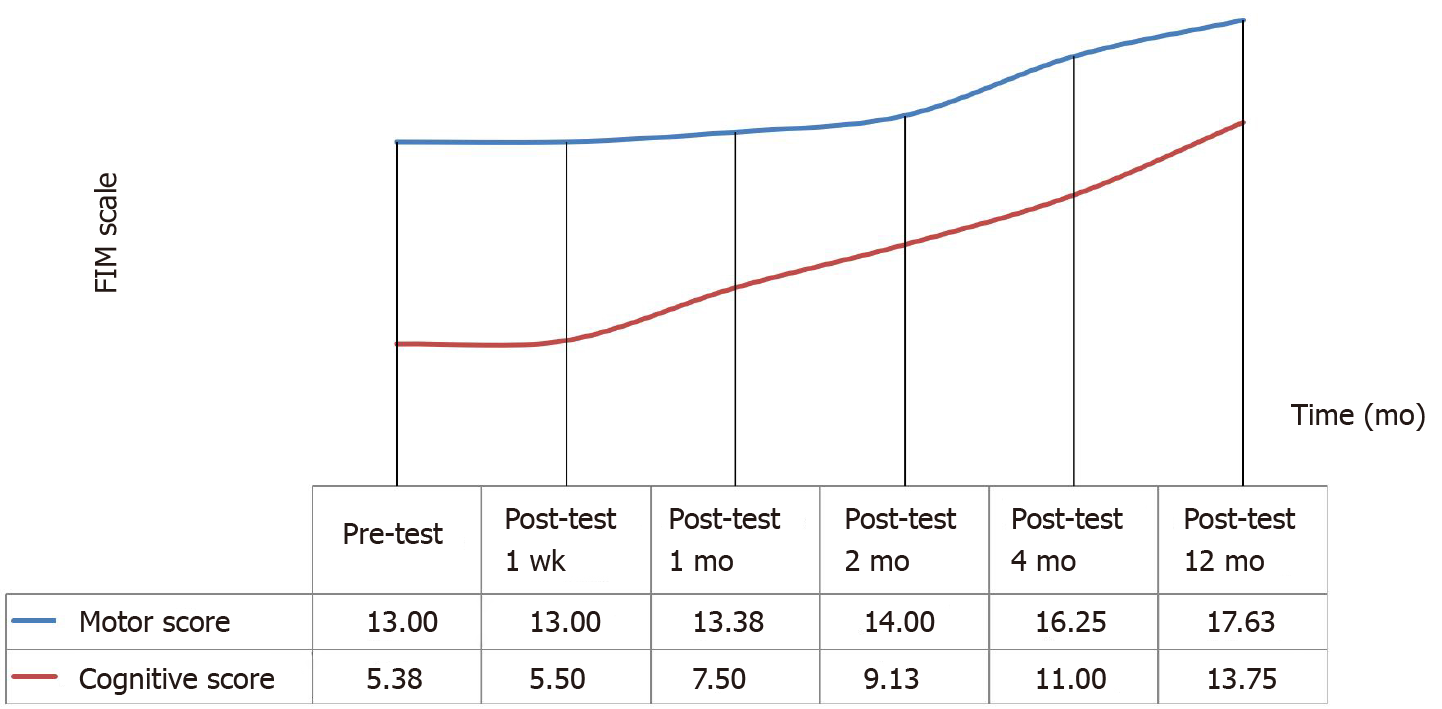
FIM Scores: The FIM scores showed significant improvement in terms of quality of life. There was a continuous increase in the FIM Motor and Cognitive scores of patients following the operation. According to the analysis in Table 5, the difference in pre- and postoperative FIM Motor scores of the participants was statistically significant (χ2 = 24.583, P < 0.001). Wilcoxon Signed Rank Test was performed between the binary measurements to identify the differences between variables. The analysis yielded no difference between baseline and one-week posttest scores (z = 0.000, P = 1.00), nor between one-week and one-month (z = -1.00, P = 0.32), one-month and two-month (z = -1.342, P = 0.18), two-month and four-month scores (z = -1.841, P = 0.07). However, a statistically significant difference was noted between four-month and twelve-month scores (z = -2.226, P = 0.026). Concluding, increases in FIM Motor scores were not significant until the second month after the operation (Figure 1 and Table 5).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Pre-test | 8 | 13.00 | 0.00 | 2.69 | 24.583 | 5 | 0.000 |
| Post-test 1 wk | 8 | 13.00 | 0.00 | 2.69 | |||
| Post-test 1 mo | 8 | 13.38 | 1.06 | 2.88 | |||
| Post-test 2 mo | 8 | 14.00 | 2.45 | 3.25 | |||
| Post-test 4 mo | 8 | 16.25 | 7.23 | 4.19 | |||
| Post-test 12 mo | 8 | 17.63 | 9.10 | 5.31 |
According to the analysis in Table 6, the difference in the pre- and postoperative FIM Cognitive scores of the participants was statistically significant (χ2 = 37.500, P < 0.001). Wilcoxon Signed Rank Test was performed between the binary measurements to identify the differences between variables. The analysis yielded no difference between baseline and one-week posttest scores (z = -1.00, P = 0.32), but significant differences between one-week and one-month (z = -2.207, P = 0.027), one-month and two-month (z = -2.384, P = 0.017), two-month and four-month (z = - 2.552, P = 0.011), and four-month and twelve-month scores (z = -2.521, P = 0.012). Concluding, increases in FIM Cognitive scores were not significant until the first week after the operation (Figure 1 and Table 6).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Pre-test | 8 | 5.38 | 1.06 | 1.63 | 37.500 | 5 | 0.000 |
| Post-test 1 wk | 8 | 5.50 | 1.41 | 1.75 | |||
| Post-test 1 mo | 8 | 7.50 | 2.45 | 2.88 | |||
| Post-test 2 mo | 8 | 9.13 | 3.83 | 3.88 | |||
| Post-test 4 mo | 8 | 11.00 | 5.68 | 5.00 | |||
| Post-test 12 mo | 8 | 13.75 | 6.23 | 5.88 |
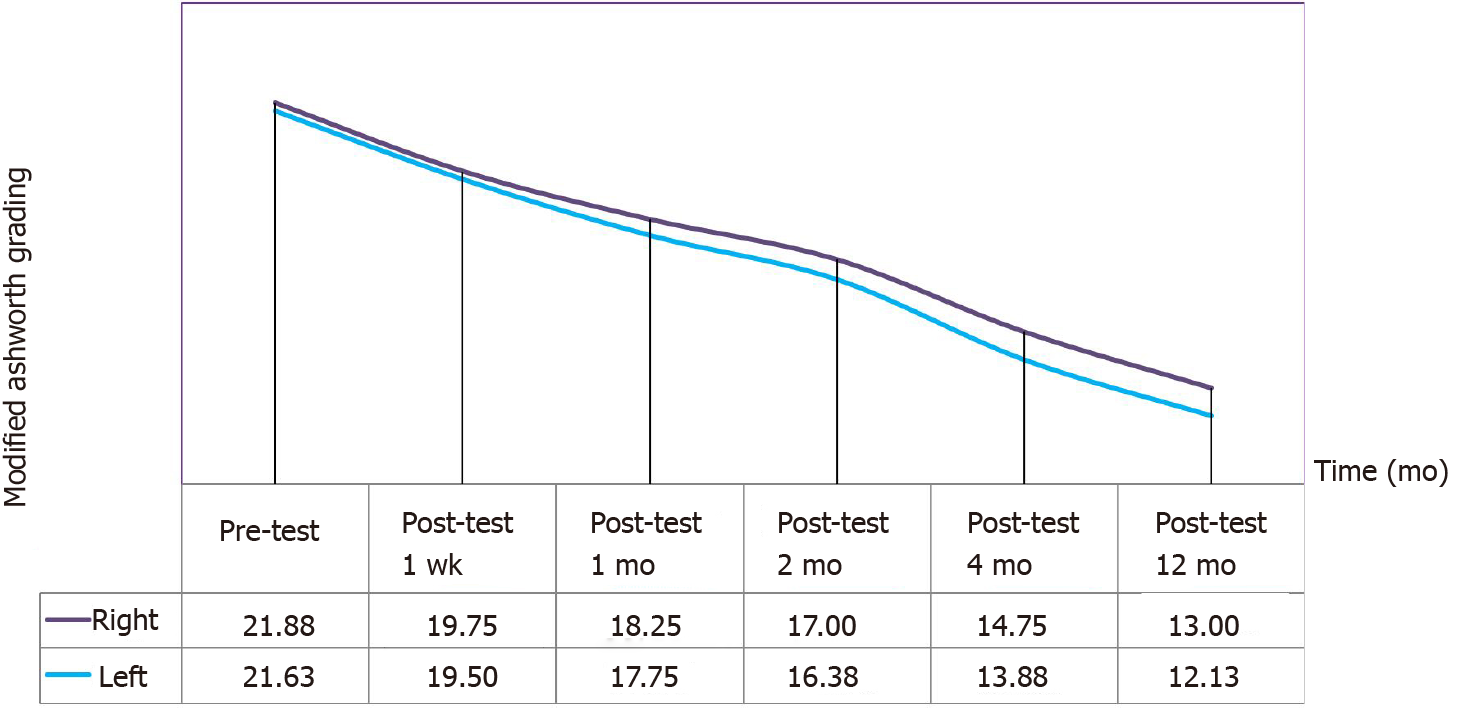
MA Scores: A continuous decrease was observed in MA right and left scores of the patients after the operation. According to the analysis in Table 7, the differences in the pre-and postoperative MA right scores of the participants was statistically significant (χ2 = 38.875, P < 0.001). Wilcoxon Signed Rank Test was performed between the binary measurements to identify the differences between variables. The analysis yielded significant differences between baseline and one-week posttest (z = -2.060, P = 0.039), one-week and one-month (z = -2.555, P = 0.011), one-month and two-month (z = -2.530, P = 0.011), two-month and four-month (z = -2.530, P = 0.011), and four-month and twelve-month scores (z = -2.533, P = 0.012). Concluding, MA right scores continued to decrease significantly until the first year after the operation (Figure 2 and Table 7).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Pre-test | 8 | 21.88 | 2.17 | 5.81 | 38.875 | 5 | 0.000 |
| Post-test 1 wk | 8 | 19.75 | 3.28 | 5.06 | |||
| Post-test 1 mo | 8 | 18.25 | 3.24 | 3.94 | |||
| Post-test 2 mo | 8 | 17.00 | 3.59 | 3.19 | |||
| Post-test 4 mo | 8 | 14.75 | 3.69 | 1.94 | |||
| Post-test 12 mo | 8 | 13.00 | 4.24 | 1.06 |
According to the analysis in Table 8, the differences in the pre- and postoperative MA left scores of the participants was statistically significant (χ2 = 38.741, P < 0.001). Wilcoxon Signed Rank Test was performed between the binary measurements to identify the differences between variables. The analysis yielded significant differences between baseline and one-week posttest (z = -2.032, P = 0.042), one-week and one-month (z = -2.338, P = 0.017), one- month and two-month (z = -2.527, P = 0.012), two-month and four-month (z = -2.527, P = 0.012), and four-month and twelve-month scores (z = -2.539, P = 0.011). Concluding, MA left scores continued to decrease significantly until the first year after the operation (Figure 2 and Table 8).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Pre-test | 8 | 21.63 | 2.83 | 5.75 | 38.741 | 5 | 0.000 |
| Post-test 1 wk | 8 | 19.50 | 3.21 | 5.066 | |||
| Post-test 1 mo | 8 | 17.75 | 2.96 | 4.06 | |||
| Post-test 2 mo | 8 | 16.38 | 3.02 | 3.13 | |||
| Post-test 4 mo | 8 | 13.88 | 3.40 | 1.94 | |||
| Post-test 12 mo | 8 | 12.13 | 3.64 | 1.06 |
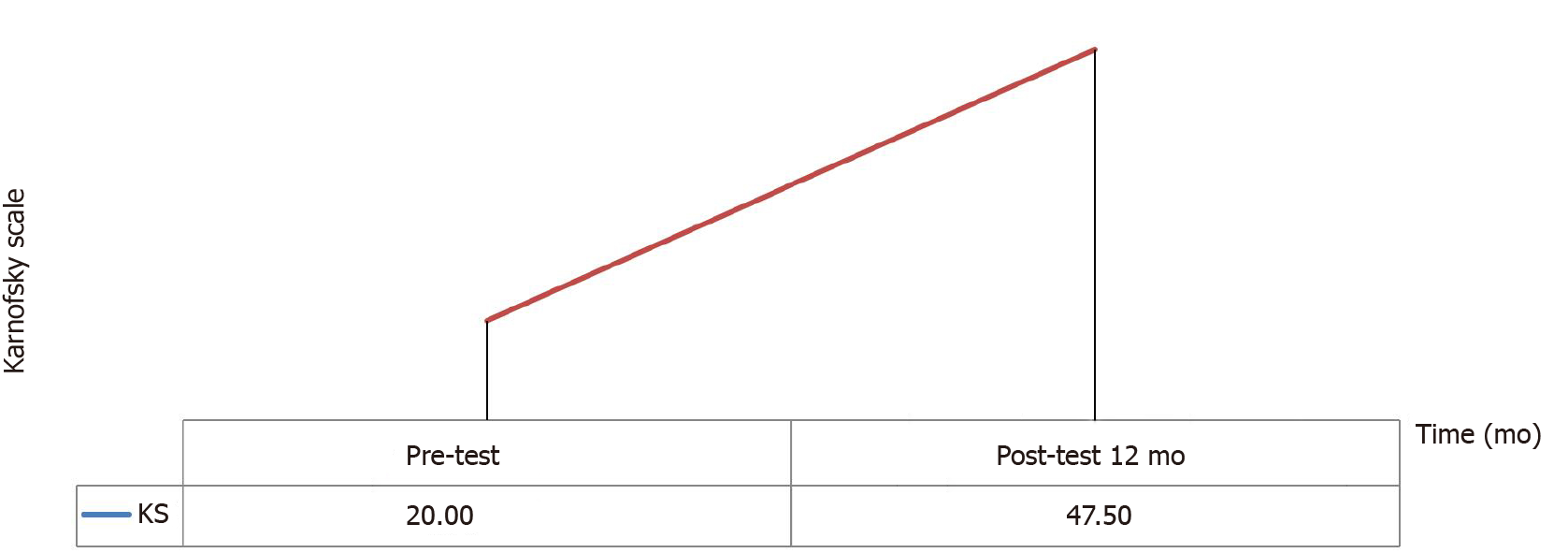
Karnofsky Scores: An increase was observed in the Karnofsky scores of the patients after the operation. According to the analysis in Table 9, a significant difference was noted between baseline and one-year posttest scores (z = -2.546, P = 0.01) (Figure 3 and Table 9).
| Karnofsky scale | Ranks | n | Mean rank | Sum of ranks | z | P value |
| Pre-test-Post-test 12 mo | Negative ranks | 0 | 0.00 | 0.00 | -2.565 | 0.010 |
| Positive ranks | 8 | 4.50 | 36.00 | |||
| Ties | 0 | |||||
| Total | 8 |
The potential disabilities to be caused by HIE can be reduced by acute therapies early after HIE and by restorative therapies during the months or years following HIE for promoting natural repair. However, there has been no specific therapy to yield particular recovery. In addition to single therapies, some options of combinations have been considered, often including moderate hypothermia, to potentially obtain better outcomes[17]. Meanwhile, the regenerative and reparative potentials of stem cells have suggested their transplantation as an alternative in the treatment of neurological disorders (e.g., stroke, spinal cord injury, etc.)[18,19]. Among the sources for these cells are neural stem/progenitor cells derived from fetal tissue, induced pluripotent stem cells, embriyonic stem cells, and MSCs[18]. The latter are multipotent progenitor cells that have a self-renewal property and the ability to differentiate into various mesodermal tissues ranging from bone and cartilage to cardiac muscle[20]. Previously, BM was considered a good candidate as a source of MSCs. However, due to its invasive nature and decreased proliferation and differentiation capacity with advanced age, alternative sources were pursued. Fetal-derived MSCs, which are more primitive and have less immune reactivity, have recently been suggested as better alternatives for BM-MSCs. WJ, which is the primitive connective tissue between the umbilical vessels and the amniotic membrane, protects these vessels from pressure and torsion. During embryogenesis, hematopoietic and mesenchymal cells migrate through the WJ, and some of them become trapped, making this tissue a good source of MSCs[21-23].
Despite the promising findings in favor of stem/progenitor cells in HIE, cellular therapy remains in the early stage for human practice[24]. Miao et al[14] found that UC-MSC given IT yielded functional improvements in 47 patients with various diseases (e.g., spinal cord injury, cerebral palsy, etc.). Some recent studies have also suggested WJ-MSC therapy to be promising for patients with neurological diseases, including stroke[12]. Allogeneic WJ-MSCs are shown to demonstrate significant positive impact, even when given via other routes, including intracerebral[9]. The current study found that multiple (4 doses in total for 2 mo at two-week intervals) triple-route (IV, IT, and IM) implantations of allogeneic WJ-MSCs are associated with safety and potential functional recovery.
To the best of our knowledge, this study is the largest to perform multiple triple-route WJ-MSC administration on HIE patients. Also, this is the first time allogeneic WJ-MSC treatment is evaluated in this patient group[12]. When applied at a dose of 1 × 106/kg IV, IT, and IM, WJ-MSC yielded mild and sparse side effects, seen only in 5 of the patients and lasting only for 24 h.
Chronic stage HIE patients often show continually increasing dysfunction, contrary to the improvement noted in the patients of in this study throughout the first year after the operation. Considering the limited number of choices for functional recovery, chronic stage disease is crucial. Normally, recovering from such dysfunctions progresses in a bimodal form, with spontaneous improvements during the first couple of months, followed by a significant rise in negative conditions within the first year after onset.
Among the findings obtained here, the most significant was the 12-point increase in FIM Motor scores, which could prove substantial if confirmed by further research. Also, at baseline, 14.28% of patients had an FIM Motor score of 91, which increased to 14.70% in the first month and 19.37% in the first year (Table 5).
The potential effects of MSCs on neurological diseases have also been marked in animal studies, and is thought to stem from some of their properties, such as restoring cellular energy, promoting neurogenesis, improving angiogenesis, and dampening inflammatory response. The 1-year sustained recovery in functional indicators demonstrated here is in line with other research on HIE[25].
The patients included in this study also had significant improvement in FIM Cognitive scores over the course of a year, indicating long-term impact on brain functions. While suggesting a promising choice for recovery in chronic stage HIE, these findings still need to be confirmed by further research, preferably of a controlled nature. In addition, such studies could measure the outcomes that are specific to certain modalities, ensuring detailed assessment regarding improvement.
This study had certain strengths in terms of its population, materials, and methods. Considering the targeted patient group, the specificity of the sample was a significant strength, in that the patients constitute a population with major dysfunction and limited options for recovery. Using allogeneic cells thanks to the nature of MSCs does not require immunosuppression and enables some treatment protocols, those that could be largely practiced on HIE patients. Using only 3 passages to harvest cell cultures was another strength, since some of the desired properties of MSCs are negatively affected by higher numbers of cell divisions, which is unavoidable when using more passages. Finally, the safety criteria used here were quite extensive in terms of both time and scope.
The study also had certain limitations, among which the most notable were the dominant focus over safety and the lack of control, as the latter complicates the interpretation of improved results. Though favorable, the findings are possibly influenced by other variables, such as growth factors and anti-inflammatory elements, perhaps even exosomes. Restorative therapies are known to aid desirable recovery outcomes, but were unfortunately lacking here.
Recently, cell therapies, particularly WJ-MSCs, have been paving the way for novel treatment protocols for preventing ischemic brain damage. However, clinical use of stem cell therapy remains conflicted and the root of its efficacy is yet to be fully clarified. Several variables still need to be elucidated, such as the mediators between cells, the optimal type and time of therapy, and the ideal patient characteristics. Thus, future multi-center studies with larger populations are needed to verify the safety and efficacy outcomes obtained here, along with an optimization for the treatment protocol in question.
To date, hypoxic ischemic encephalopathy (HIE) is refractory, including after cardiopulmonary resuscitation, hemorrhagic shock and cerebral infarction etc.
Limited treatment options exist for patients with HIE and substantial functional deficits. Thus, further clinical research investigations on this subject could be valuable.
The current study examined safety and preliminary efficacy estimates of allogeneic mesenchymal stem cells (MSCs) in this population.
Entry criteria included HIE ≥ 6 mo prior, substantial impairment and disability. Enrollees received intrathecal, intramuscular, and intravenous administrations of Wharton’s jelly–derived MSCs at a target dose of 1 × 106/kg for each application route (twice a month for 2 mo).
Treatment was safe based on serial neurological and functional exams, laboratory tests, electroencephalographies, and magnetic resonance imaging.
Therapeutic administration of stem cells has a theoretical role in the treatment of HIE. Although promising results from many publications have been reported, there is still no consensus on which cellular therapy should be administered to which patient at what time after HIE. There seems to be a need for a tremendous amount of work to elucidate the underlying mechanisms of how MSCs interact with damaged host tissues and how this interaction results in a cascade of events that lead to some functional neuronal recovery.
These findings suggest that quality of the cells, optimization of the cell dose, standardization of the cell processing, the timing, route of administration and patient selection as well as the role of clinical experience of the physcisian are critical to the success of stem cell therapy in HIE patients.
Manuscript source: Invited manuscript
Specialty type: Neurosciences
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cazorla E, Yao D S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2019;173:1-17. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Brownlee NNM, Wilson FC, Curran DB, Lyttle N, McCann JP. Neurocognitive outcomes in adults following cerebral hypoxia: A systematic literature review. NeuroRehabilitation. 2020;47:83-97. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Disdier C, Stonestreet BS. Hypoxic-ischemic-related cerebrovascular changes and potential therapeutic strategies in the neonatal brain. J Neurosci Res. 2020;98:1468-1484. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Yang T, Li S. Efficacy of different treatment times of mild cerebral hypothermia on oxidative factors and neuroprotective effects in neonatal patients with moderate/severe hypoxic-ischemic encephalopathy. J Int Med Res. 2020;48:300060520943770. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Nitkin CR, Rajasingh J, Pisano C, Besner GE, Thébaud B, Sampath V. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr Res. 2020;87:265-276. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Dailey T, Metcalf C, Mosley YI, Sullivan R, Shinozuka K, Tajiri N, Pabon M, Acosta S, Kaneko Y, van Loveren H, Borlongan CV. An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside. J Clin Med. 2013;2:220-241. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Nabetani M, Shintaku H, Hamazaki T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2018;83:356-363. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Qin X, Cheng J, Zhong Y, Mahgoub OK, Akter F, Fan Y, Aldughaim M, Xie Q, Qin L, Gu L, Jian Z, Xiong X, Liu R. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front Mol Neurosci. 2019;12:88. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Zhang L, Li Y, Romanko M, Kramer BC, Gosiewska A, Chopp M, Hong K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012;1489:104-112. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Joerger-Messerli MS, Marx C, Oppliger B, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal Stem Cells from Wharton's Jelly and Amniotic Fluid. Best Pract Res Clin Obstet Gynaecol. 2016;31:30-44. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Mesenchymal Stem Cells: The Magic Cure for Intraventricular Hemorrhage? Cell Transplant. 2017;26:439-448. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Kabataş S, Civelek E, İnci Ç, Yalçınkaya EY, Günel G, Kır G, Albayrak E, Öztürk E, Adaş G, Karaöz E. Wharton's Jelly-Derived Mesenchymal Stem Cell Transplantation in a Patient with Hypoxic-Ischemic Encephalopathy: A Pilot Study. Cell Transplant. 2018;27:1425-1433. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Okur SÇ, Erdoğan S, Demir CS, Günel G, Karaöz E. The Effect of Umbilical Cord-derived Mesenchymal Stem Cell Transplantation in a Patient with Cerebral Palsy: A Case Report. Int J Stem Cells. 2018;11:141-147. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Miao X, Wu X, Shi W. Umbilical cord mesenchymal stem cells in neurological disorders: A clinical study. Indian J Biochem Biophys. 2015;52:140-146. [PubMed] [Cited in This Article: ] |
| 15. | Thorpe ER, Garrett KB, Smith AM, Reneker JC, Phillips RS. Outcome Measure Scores Predict Discharge Destination in Patients With Acute and Subacute Stroke: A Systematic Review and Series of Meta-analyses. J Neurol Phys Ther. 2018;42:2-11. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Huang H, Young W, Chen L, Feng S, Zoubi ZMA, Sharma HS, Saberi H, Moviglia GA, He X, Muresanu DF, Sharma A, Otom A, Andrews RJ, Al-Zoubi A, Bryukhovetskiy AS, Chernykh ER, Domańska-Janik K, Jafar E, Johnson WE, Li Y, Li D, Luan Z, Mao G, Shetty AK, Siniscalco D, Skaper S, Sun T, Wang Y, Wiklund L, Xue Q, You SW, Zheng Z, Dimitrijevic MR, Masri WSE, Sanberg PR, Xu Q, Luan G, Chopp M, Cho KS, Zhou XF, Wu P, Liu K, Mobasheri H, Ohtori S, Tanaka H, Han F, Feng Y, Zhang S, Lu Y, Zhang Z, Rao Y, Tang Z, Xi H, Wu L, Shen S, Xue M, Xiang G, Guo X, Yang X, Hao Y, Hu Y, Li J, Ao Q, Wang B, Lu M, Li T. Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017). Cell Transplant. 2018;27:310-324. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Allen KA, Brandon DH. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs Rev. 2011;11:125-133. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92-115. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Teng YD, Kabatas S, Li J, Wakeman DR, Snyder EY, Sidman RL. Functional multipotency of neural stem cells and its therapeutic implications. In: Ulrich H, editor. Perspectives of Stem Cells. Springer Dordrecht Heidelberg London New York Springer Science+Business Media B.V.; 2010; 255-270. [DOI] [Cited in This Article: ] |
| 20. | Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248-269. [PubMed] [Cited in This Article: ] |
| 21. | Witkowska-Zimny M, Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton's jelly, amnion and chorion. Cell Mol Biol Lett. 2011;16:493-514. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Wang XY, Lan Y, He WY, Zhang L, Yao HY, Hou CM, Tong Y, Liu YL, Yang G, Liu XD, Yang X, Liu B, Mao N. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood. 2008;111:2436-2443. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and Feasibility of Autologous Mesenchymal Stem Cell Transplantation in Chronic Stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13:14-19. [PubMed] [Cited in This Article: ] |