Selectivity and Sensitivity: The Electron Capture Detector (ECD), Its Unique Inventor James Lovelock (1919–2022), and GAIA
The electron capture detector (ECD) is among the original classical detectors for gas chromatography (GC). It is highly selective and sensitive for electron-absorbing compounds, especially those containing halogens. The ECD was developed in the 1960s by James Lovelock (1919–2022), who passed away earlier this year. The ECD is among the early detectors that is used in a relatively unmodified form today. The ECD was crucial in the discovery of chlorofluorocarbons (CFCs) in the upper atmosphere, ultimately leading to the international agreements limiting their use and reducing the ozone hole. In this installment, we review the ECD and its principles of operation and discuss the general tradeoffs in detection between selectivity, ease of use, and sensitivity. We also look at the unique life and work of Lovelock, both inventor of the ECD and one of the earliest scientists to bring attention to climate change through GAIA, which explained his theory that the Earth acts as a massive living organism.
This summer, I was watching the news show “Sunday Today,” and heard a name familiar to many gas chromatographers: James Lovelock. To chromatographers, Lovelock invented the electron capture detector (ECD) for gas chromatography (GC). He was featured in the weekly “Life Well Lived” segment, where the show features people who are not widely known to the public but made contributions to public life (1). The segment describes how the ECD and Lovelock’s own work provided major influences on climate science today, nearly 60 years later.
Detection in GC remains unique in analytical chemistry because of the large number of customized detectors. For early workers, detection was especially challenging because the detector was required to be sensitive, selective, and able to detect a low concentration or mass of analytes in a rapidly moving stream of carrier gas. Interestingly, many of these early detectors, including flame ionization detectors (FIDs), thermal conductivity detectors (TCDs), and ECDs remain widely used today, along with myriad additional ionization, physical property, and spectrometric detectors. Schug and McNair provided a summary and “grade sheet” for commonly used GC detectors (2). The ECD was graded A+ for limit of detection (LOD) and specificity, but it was given a C grade for qualitative speciation, linear range, and robustness, and a D grade for universal response.
A summary of the basic principles and operation for the ECD is provided in textbooks on GC and available in ChromAcademy, LCGC North America’s online learning platform (3–5). Overall, the ECD provides a unique combination of selectivity and sensitivity. Implied by its name, the ECD offers selectivity for compounds that include atoms that are favorable for attracting or capturing an electron. Recalling general chemistry, note that elements on the right and up in the Periodic Table tend to attract electrons. These include the most common heteroatoms, especially halogens. The ECD is highly selective for compounds containing these elements, and by contrast, generates little or no signal for common hydrocarbons. The ECD presents a useful complement and contrast to the FID, which gives strong signals for hydrocarbons.
Highly selective detectors, in general, offer greater sensitivity for those analytes for which they are selective. The ECD is noted to be the most sensitive of the classical detectors, with detection limits easily in the part- per-billion (ppb) concentration or picogram mass range. Finally, highly sensitive detectors, including the ECD, have very specific requirements for obtaining the expected high sensitivity. In the case of the ECD, the carrier and makeup gases must have scrupulous high purity, samples should be especially clean, and the operating environment should be free of contaminants containing electron absorbing atoms. Together, these operational factors lead to the grades shown for the ECD by Schug and McNair.
Figure 1 shows a schematic diagram of a typical ECD. The operating principle, while not fully understood, first involves the production of electrons from a radioactive source, typically 63Ni. For safety, a sealed source is used and usually the detector manufacturer will have a blanket radioactive isotope license in case the end user does not. Radioisotope licensing was an early barrier for ECD users. If you ever need to dispose of an ECD, you should consult with the manufacturer, a licensed health physicist, or radiation safety officer for proper disposal procedures.
FIGURE 1: Schematic of electron capture detector (ECD).
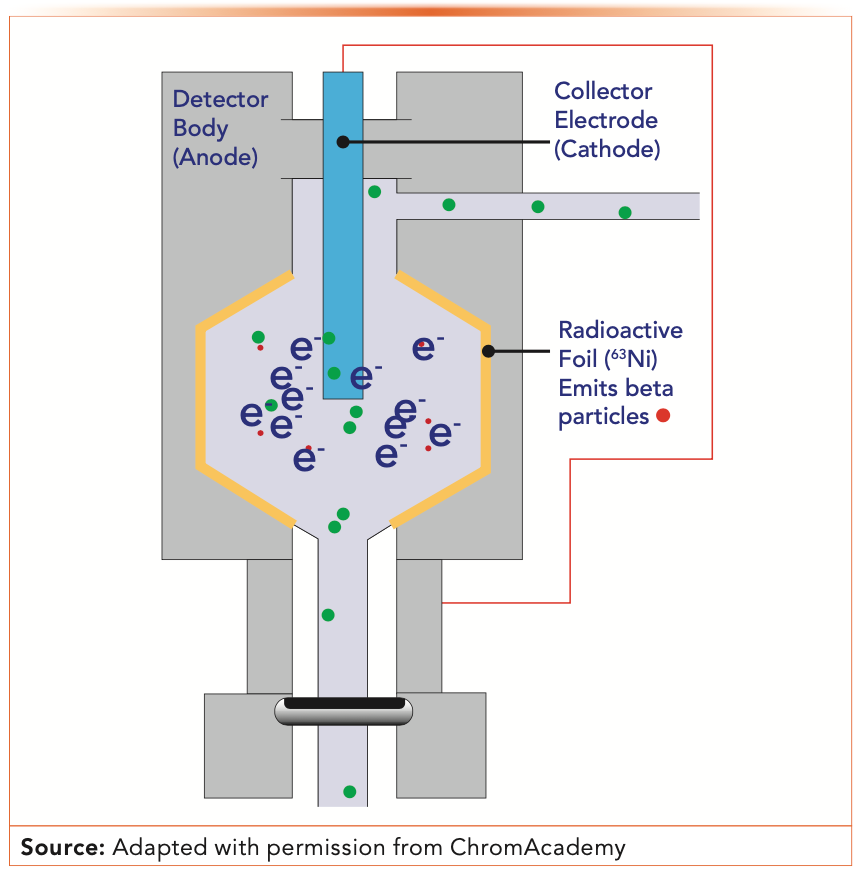
Unique among ionization detectors, the ECD measures a reduction in the electrical current rather than production of a current. Electrons produced in the radioactive source ionize carrier gas, usually nitrogen, molecules flowing through the detector cell. If the carrier gas is helium or hydrogen, nitrogen makeup gas is used. Electrons produced by the ionized nitrogen migrate to the anode under a fixed voltage, generating a steady electrical current. If electron-absorbing species pass into the detector cell, they absorb electrons, resulting in a reduction in the standing current.
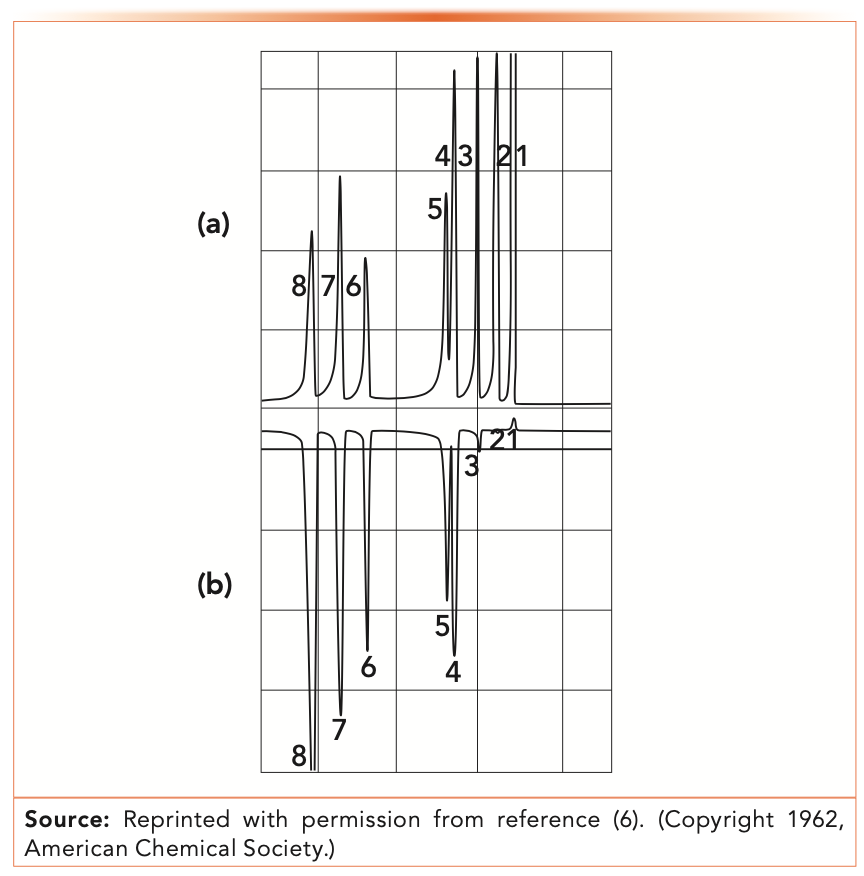
Figure 2 shows an early example of ECD selectivity from Lovelock’s original work in 1960 (6). The top chromatogram shows the separation of eight compounds using a classical argon detector (not commonly used today). The bottom chromatogram shows the separation of the same eight compounds using the ECD. Note the stronger argon detector signals for the hydrocarbon and fluorinated compound, for the singly-chlorinated benzene, and the strong ECD signals for the multiply chlorinated compounds.
FIGURE 2: (a) Argon detector and (b) ECD chromatograms of a mixture of hydrocarbons and halocarbons. 1. cyclohexane; 2. fluorobenzene; 3. chlorobenzene; 4. m- and p-dichlorobenzene; 5. o-dichlorobenzene; 6. 1,3,5-trichlorobenzene; 7. 1,2,4-trichlorobenzene; and 8. 1,2,3-trichlorobenzene.
In 2007, Ettre and Morris provided a thorough discussion “The Saga of the Electron Capture Detector” in LCGC North America (7). They trace the developments that led to the ECD beginning in 1948, showing a fascinating scientific story that began with studying a different problem (whether drafts of cold air in a room really do cause illnesses) for which Lovelock developed an anemometer that worked by disturbing the slow flow of positive ions from a radon source. This anemometer was so sensitive that the signal was disturbed by small amounts of cigarette smoke in the atmosphere, capturing electrons, although at the time this was seen as a problem and not of any interest. Recall that smoking was much more prevalent in the 1950s than it is today. In my own career working with modern ECDs, I have observed deflections in ECD signals because of the presence of cigarette smoke odors. The full story, presented by Ettre and Morris, of how this observation ultimately led to today’s ECD is well worth the read.
The Science Museum of London also has an early ECD and other equipment, including Lovelock’s own home-made gas chromatograph on display and an extensive treatment of Lovelock’s life and career, along with Lovelock’s entire personal archive of documents and letters (8).
Lovelock, Atmospheric Chemistry, and GAIA
Lovelock’s prolific scientific career, spanning seven decades, included many more contributions than inventing the ECD for use in GC. As with many of the early gas chromatographers, his career started in other areas. His first publication in 1941 related to the use of hypochlorite spraying for disinfectants (9). Then, he had a prolific publication record in the general area of disinfectants, sports training, prevention of the common cold, and other medical topics through the remainder of the 1940s and early 1950s. After earning his PhD in medicine in 1948, a shift toward instrumentation and measuring devices was seen throughout the 1950s. Through the 1950s, his medical research shifted to many aspects of preservation and chemical effects on red blood cells and some interestingly titled articles related to the re-animation of rats and mice frozen to 0 °C.
As Ettre and Martin describe, in the 1950s, Lovelock’s laboratory was in close proximity to A.J.P Martin and A.T. James, who invented GC and they became collaborators, with Martin suggesting to Lovelock that he develop a highly sensitive detector that was operable by average chemists. They noted that the most sensitive detectors of the day were not easily operated by most chemists. The first mention of GC in the title of an article written by Lovelock occurred in 1958. In the article, he discussed an early detector, not the ECD but the argon ionization detector, which like the ECD used a radioactive source to produce electrons that ultimately ionized analytes. It was a nearly universal detector, used for many applications that were ultimately supplanted by the FID, which did not require a radioactive source (10). Using the argon ionization detector, Lovelock then published numerous papers on the ionization mechanisms of organic compounds in the vapor phase, most often using GC with the argon ionization detector.
Following the initial invention and publication of the ECD (6), Lovelock worked with Zlatkis to commercialize the detector, founding a company called Ionics Research, which provided ECDs to PerkinElmer and the Wilkens Instrument and Research Company, which later became the Chromatography Division of Varian bought in 2010 by Agilent Technologies. In 1962, coincidentally with the publication of the classic book Silent Spring by Rachel Carson, the first papers demonstrating ECD of pesticides at the picogram level were being published; although, as pointed out by Ettre and Morris, the ECD is often incorrectly cited, including in the “Sunday Today” segment, as one of the techniques on which Carson’s findings were based (11). However, there is clear connection between Lovelock’s work, the ECD, the early environmental movement, and today’s challenges with climate change.
Using the ECD, Lovelock published the first work demonstrating the presence of chlorofluorocarbons (CFCs) in the atmosphere over the Atlantic Ocean between England and Antarctica (12). This work demonstrated the presence of Freon-11 and other compounds resulting from the use of aerosol cans and refrigerants. Roland and Molina then used this work in developing the theory that halocarbons in the atmosphere were reacting with stratospheric ozone in a chain reaction, causing the depletion of ozone and the famous “ozone hole” (13). Roland, Molina, and Crutzen shared the Nobel Prize in 1995, and this work resulted in international treaties limiting the use of halocarbons.
We close with GAIA, a fundamental aspect of climate science today, that was mocked by most when introduced by Lovelock in the 1970s. Much like the seeming lack of interest in the 1950s in a detector that was affected by cigarette smoke in the room, GAIA, named for the Greek goddess of the personification of Earth, proposed that the Earth is a single self-sustaining organism, and this idea was universally rejected by the scientific community of the day (14). The basic principles and problems described by Lovelock in his original work remain debated in climate discussions today. With the invention of the ECD, its use in discovering CFCs in the atmosphere, work in planetary, medical, space and climate science, James Lovelock was not just a chromatographer but a true Renaissance man of science.
References
(1) Sunday Today, Life Well Lived. https://www.today.com/video/dr-james- lovelock-scientist-who-created-gaia-theory-dies-at-103-145164869912 (Accessed October, 2022).
(2) K.A. Schug and H.M. McNair, LCGC North Am. 33(1), 24–33 (2015).
(3) H.M. McNair, J.M. Miller, and N.H. Snow, Basic Gas Chromatography (John Wiley and Sons, Hoboken, NJ, 2019), pp. 131–134.
(4) C.F. Poole, Ed., Gas Chromatography (Elsevier, Amsterdam, The Netherlands, 2nd ed., 2021), pp. 343–358.
(5) ChromAcademy, GC Detectors. https://www.chromacademy.com/channels/gc-training-courses/instrumentation/gc-detectors/ (accessed October 2022).
(6) J.E. Lovelock and S.R. Lipsky, J. Am. Chem. Soc. 82, 431–433 (1960).
(7) L.S. Ettre and P.J.T. Morris, LCGC North Am. 25(2), 164–178 (2007).
(8) Science Museum, Something in the Air: James Lovelock and Atmospheric Pollution. https://www.sciencemuseum.org.uk/ objects-and-stories/chemistry/something-air-james-lovelock-and-atmospheric-pollution (accessed October 2022).
(9) J.E. Lovelock and O.M. Lidwell, The Lancet 238(6172), 746 (1941).
(10) J.E. Lovelock, J. Chromatogr. A. 1, 35–46 (1958).
(11) R. Carson, Silent Spring (Houghton-Mifflin, Boston, MA, 1962).
(12) J.E. Lovelock, R.J. Maggs, and R.J. Waade, Nature (London) 241, 194–196 (1973).
(13) F.S. Roland and M.J. Molina, Rev. Geophys. Space Phys. 13, 1–36 (1975).
(14) J.E. Lovelock, GAIA: A New Look at Life on Earth (Oxford University Press, Oxford, United Kingdom, 1979).
ABOUT THE AUTHOR
Nicholas H. Snow is the Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, and an Adjunct Professor of Medical Science. During his 30 years as a chromatographer, he has published more than 70 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction. Direct correspondence to: LCGCedit@mmhgroup.com


Inside the Laboratory: The Chromatography Laboratory at the University of Rouen
April 18th 2024In this edition of “Inside the Laboratory,” Pascal Cardinael and Valérie Agasse of the University of Rouen in Mont‑Saint-Aignan, France, discuss their laboratory’s work with miniaturizing gas chromatography (GC) columns and systems to improve on-site air analysis of volatile organic compounds (VOCs).