Abstract
Background:
This study aimed at evaluating the effect of the extract and essential oil of Zataria multiflora on reducing phenotypes and genotypes of biofilm formation in clinical isolates of Staphylococcus epidermidis.Methods:
The ability to produce biofilm was evaluated by microtiter plate (MtP) for phenotype and through the presence of icaADB and aap by PCR for genotype among the 153 clinical isolates of Staphylococcus epidermidis. The impact of ethanol extract and essential oil of Zataria multiflora were measured on biofilm formation in phenotypic evaluation based on MtP and on the expression of ica operon and aap by real time-PCR.Results:
The results showed that 71.2% of isolates were able to produce biofilms. The PCR results showed that 52.2% and 88.9% of the isolates had icaABD and aap, respectively. In addition, the relative mRNA expression of icaA, icaD, and aap genes were significantly reduced compared to the negative control after treating the Staphylococcus epidermidis RP62A with subMIC concentration of essential oil and extract (P < 0.001).Conclusions:
Given the significant inhibitory effect of the extract and essential oil of Zataria multiflora on biofilm formation, it seems that these substances are good options for studies related to controlling biofilm formation in Staphylococcus epidermidisKeywords
1. Background
Staphylococcus epidermis is the most frequent cause of implanted medical device or catheter-related infections and it has caused an increase in resistance to different types of antibiotics in hospitals through its most significant virulence factor, i.e. biofilm production. Polysaccharide intercellular adhesive (PIA) is a product of the ica operon and is strongly associated with staphylococcal cell surface and the main composition, which mediates cell to cell adhesion. The ica operon contains ica ADBC genes, which are required for PIA synthesis (1). Other pathways also contribute to biofilm formation in Staphylococcus epidermis independent of the ica operon, such as the biofilm accumulation associated protein (Aap) forming fibrillary structures on the cell surface (2).
Zataria multiflora is a plant from the Labiatae family that grows in Iran, Pakistan, and Afghanistan. The traditional uses of Zataria multiflora include antiseptics, anesthetics, and antispasmodic (3). In this plant, the most antibacterial activity is related to essential oil with compounds including thymol and/or carvacrol, phenolic derivatives. The anti-biofilm activity of Zataria multiflora was reported against Pseudomonas aeroginosa and Listeria monocytogenes, and antibacterial activity of Zataria multiflora was shown against Staphylococcus epidermidis strains, yet there is no study about the role of Zataria multiflora on inhibition of biofilm formation in Staphylococcus epidermidis isolates (4).
Many studies have investigated the effect of different substances on biofilm production in Staphylococcus epidermis, including silver colloidal nanoparticles and Cassia alata leaves (5). The effect of Zataria multiflora Boiss on biofilm production in Staphylococcus epidermis has not been addressed in the literature, although its inhibitory effects on growth and biofilm production has been studied in other bacteria (6).
2. Objectives
The present study was conducted to examine the in-vitro effect of Zataria multiflora Boiss extract and essence on biofilm production in Staphylococcus epidermis.
3. Methods
3.1. Sample Collection
A total of 153 S. epidermidis clinical isolates were obtained between April 2014 and January 2015 from Baqiyatallah Hospital, Tehran, Iran. The isolates were from blood, wound, bronchoalveolar lavage, urine, cerebral spinal fluid, tracheal, sputum, throat, and eye samples. Identification included biochemical tests, such as catalase, coagulase, PYR, and urea tests and susceptibility to novobiocin (7). After identification, samples were inoculated in BHI-broth at 37°C for 24 hours. Each sample was stored at -80ºC.
3.2. Antimicrobial Susceptibility Test
Antimicrobial susceptibility was performed for clindamycin (2 μg), cefoxitin (30 μg), erythromycin (15 μg), tetracycline (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), rifampicin (5 μg), linezolid (30 μg), theicoplanin (30 μg), amikacin (30 μg), and meropenem (10 μg) by the disk-diffusion method using antimicrobial disks (MAST) and Muller-Hinton agar medium (Merk, Germany) (8) and interpreted according to CLSI procedures.
3.3. Analysis of Biofilm Forming Isolates
To identify the biofilm forming isolate, the microtiter plate test (mpt) was used, with a modification of the method described by Merritt et al. (9) The BHI broth cultures were used to produce a bacterial suspension in Muller-Hinton Broth with a density of 0.5 McFarland, then 200 μL from each suspension was added to 96-well microtiter plates. The plates were incubated overnight at 37°C. After incubation, the free floating cells were removed and submerged in a tray full of distilled water. Following the washing, the plates were stained with 125 μL of 0.2% (w/v) crystal violet, and then incubated at room temperature for 15 minutes. The dye was rinsed with distilled water three times, and the plates were allowed to air-dry. Then, 200 μL of absolute ethanol was added and incubated for a further 15 minutes at room temperature. A total of 200 μL from each well was transferred to a fresh microtiter plate well. Finally, the optical density of each suspension was measured at 570 nm. Staphylococcus epidermidis RP62A was used as a positive control for the biofilm assay while the negative control consisted of all added reagents with the exception of the bacterial suspension. The test was repeated in triplicates. Mean absorbance at 570 nm ± SD was calculated. According to Merritt et al., if the optical density is higher than 0.2, the strain is considered a biofilm producer (10).
3.4. Detection of icaABD, IS256 and aap Genes
In order to study the frequency of icaABD, IS256, and aap genes among the tested isolates, the PCR method was used. Total genomic DNA extraction of S. epidermidis isolates was performed using a DNA extraction kit (GenAll Biotechnology, Korea). DNA content was determined by measuring absorbance at 260 nm. Overall, 1 µL of each DNA was added to 25 μL of mixture reaction including 10X reaction buffer, 2 mM MgCl2, 0.1 mM of the deoxynucleoside triphosphates (dNTPs), 10 ρmol of designed primers, according to Table 1 and 1.5 U of Taq DNA Polymerase (Cinnagen, Tehran, Iran) and was amplified based on PCR conditions in a DNA thermal cycler, including pre-denaturation at 95°C for five minutes, 35 cycles of denaturation at 95°C for one minute, annealing at 59°C for one minute, extension at 72°C for one minute, and final elongation at 72°C for five minutes for icaABD and IS256 genes and pre-denaturation at 94°C for five minutes, 35 cycles of denaturation at 94°C for 45 seconds, annealing at 54°C for 45 seconds, extension at 72°C for one minute, and final elongation at 72°C for five minutes for aap gene. In this study, the researchers used the IS256 gene to determine the pathogenicity of S. epidermidis strains and detected ica and aap genes to analyze the potency of biofilm formation. The PCR products were detected in 1.5% agarose gel with the use of ethidium bromide and visualized by UV trans-illumination.
Specific Primers Used for PCR
| Primer Name | Sequence (5’-3’) | Amplicon Size (bp) | Ref |
|---|---|---|---|
| icaBD | 516 | (11) | |
| F | TTATCAAT GCCGCAGTTGTC | ||
| R | GTTTAACGCG AGTGCGCTAT | ||
| IS256 | 1013 | (12) | |
| F | TGAAAAGCGAAGAGATTCAAAGC | ||
| R | ATGTAGGTCCATAAGAACGGC | ||
| Aap | 1100 | (13) | |
| F | ATGGGCAAACGTAGACAAG | ||
| R | ACCGTAAAAATCGTAATTATCTC | ||
| Gyr | 154 | (14) | |
| F | CTTATATGAGAATCCATCTGTAGG | ||
| R | AGAACAATCTGCCAATTTACC | ||
| IcaD | 197 | (15) | |
| F | CCGGAGTATTTTGGATGTATTG | ||
| R | TTGAAACGCGAGACTAAATGTA | ||
| IcaA | 186 | (16) | |
| F | TCTCTTGCAGGAGCAATCAA | ||
| R | AGGCACTAACATCCAGCA | ||
| Aap | 117 | (17) | |
| F | AGAAACAAGCTGGTCAAG | ||
| R | CTGCGTAGTTAAGAAAATC |
3.5. Plant Material
During year 2014, fresh plant leaves and seeds of Zataria multiflora were purchased from a local herbal medicinal shop in Tehran, Iran.
3.6. Preparation of the Essential Oil of Zataria multiflora
Overall 20 g of ground parts of leaves and seeds of Zataria multiflora were soaked in 500 mL of sterile distilled water and distillate by Clevenger apparatus for two hours. The yield of essential oil was stored at 4°C.
3.7. Determination of MIC and MBC Concentration by the Broth Microdilution Method
The MIC of Zataria multiflora essential oil was determined in LB broth by broth microdilution method to yield concentrations ranging from 0.25 to 10 (%, v/v). Microbial suspension of Staphylococcus epidermidis RP62A strain was justified to 0.5 MacFarland. Overall, 40 μL of microbial suspension with LB broth medium and essential oil concentration were added to each tube, and tubes were incubated at 37°C for 18 hours. The lowest concentration of essential oil with no visible bacterial growth was determined as the MIC. The MBC of essential oil was reported using the first tube showing no growth on LB agar. Finally, the sub-MIC of essential oil was determined.
3.8. Preparation the Ethanol Extract of Zataria multiflora
Leaves and seeds of Zataria multiflora were dried, ground in a grinder, and about 100 g of dry powder was extracted with 1500 mL of 70% ethanol for 48 hours. Then the extract was filtered through Wathman no 1 filter paper. The solvent of the extract was evaporated by a vacuum evaporator, and finally residues were dried and used for the experiment. The major components of Zataria multiflora extract were analyzed using a gas chromatography-mass spectroscopy (GC-MS).
3.9. Determination of MIC and MBC Concentration by Broth Microdilution Method
The MIC of Zataria multiflora extract were determined in LB broth by the microdilution method. The extract was diluted in 80% DMSO to yield concentrations ranging from 0.25 to 10 (%, w/v). Microbial suspension of Staphylococcus epidermidis RP62A strain was adjusted to 0.5 MacFarland. Overall, 40 μL of suspension bacterial with LB broth medium and extract concentrations were added to each tube and tubes were incubated at 37°C for 18 hours. The lowest concentration of extract with no visible bacterial growth was determined as the MIC. The MBC of the extract was reported using the first tube showing no growth on LB agar. Finally, the subMIC of the extract was determined.
3.10. Effect of Sub-Inhibitory Concentration of Essential Oil and Extract on Biofilm Formation
Microbial suspensions of 109 Biofilm positive strains in Muller Hinton Broth medium were adjusted to 0.5 MacFarland and 100 μL were added to each well of 96-well microtiter plate. Then, 100 μL of sub-inhibitory concentration (sub-MIC) of essential oil (9%) and extract (4 mg/mL) were dispensed in each well. Microbial suspensions and Muller Hinton Broth medium without the essential oil and extract were used as the negative control. Microplates were incubated at 37°C for 18 hours. Each assay was repeated in duplicates. Following incubation, biofilm formation was performed as described above (9).
3.11. Effect of Sub-Inhibitory Concentration of Essential Oil and Extract on the Expression of icaAD and aap Genes by Use of Real Time-PCR
The microbial suspension of Staphylococcus epidermidis RP62A in LB broth medium was adjusted to 0.5 MacFarland. Overall, 20 μL of microbial suspension with LB broth medium were separately added to tubes containing sub-MIC concentration of essential oil (9%) and extract (4 mg/mL). The microbial suspension without plant was evaluated as a negative control. After overnight incubation at 37°C, the bacterial pellet was collected using centrifuge at 5000 Xg for 20 minutes and RNA extraction was performed using the Genall kit (GenAll Biotechnology, Korea). The RNA concentration was determined by nano-drop. Complementary DNA (cDNA) was synthesized using the Thermoscientific kit. Quantitative real time PCR was performed for appointment of scale of icaAD and aap genes expression. The primers that were used in this study are shown in Table 1. One microliter of each cDNA was amplified in 20 μL of mixture reaction containing 2X cyber green mixture reaction and 10 ρmol of designed primers (Viragene, USA). The expression level of gyr gene was surveyed as an internal control. The PCR protocol was exerted by Corbet 6000 with an initial denaturation step during three minutes at 94ºC, 38 cycles of 30 seconds at 94ºC, 30 seconds at the desired annealing temperature, and 30 seconds at 72ºC. All tests were done in triplicates. The relative fold change alterations in the expression were analyzed by the Rest 2009 software.
3.12. Statistical Analysis
The Pearson chi-squared test was performed in order to explore the relationship between biofilm production and the presence of icaABD and aap genes, the correlation between biofilm formation and drug resistance, and the relationship between the presence of icaABD, aap genes, and drug resistance. P value was considered significant at < 0.05. Data analysis was performed using SPSS 16.0 (SPSS Inc., IBM, USA).
4. Results
4.1. Antibiotic Resistance
Antimicrobial susceptibility was determined using the disk diffusion method. Staphylococcus epidermidis strains showed various susceptibilities to tested chemotherapeutics (Figure 1). The strains had high resistance to erythromycin (86.2%) and lower resistance to linezolid (6.5%) (Figure 1).
The number/percentage of S. epidermidis strains resistant to selected antibiotics

4.2. Biofilm Formation
The MtP method was used to determine biofilm forming ability of isolates. Overall, 109 (71.2%) of the isolates were biofilm positive and they were used for evaluating the effect of extract and essential oil. Table 2 summarizes the antibiotic resistance pattern among biofilm producer and non-producer isolates. There was no significant discrepancy in antibiotic resistance between isolates with the ability of biofilm production and those without these properties (P > 0.05).
Frequency of Antibiotic Resistance Among Biofilm Producer and Non-Producer S. epidermidis Isolatesa
| Antibiotics | Resistant | ||
|---|---|---|---|
| Total Isolates | Biofilm Forming Isolates | Non Biofilm Forming Isolates | |
| FOX | 90 (58.8) | 50 (52.6) | 40 (69) |
| E | 132 (86.2) | 77 (81.1) | 55 (94.8) |
| DA | 116 (75.8) | 65 (68.4) | 51 (87.9) |
| T | 90 (58.8) | 52 (54.7) | 38 (65.5) |
| C | 20 (13) | 8 (8.4) | 12 (20.7) |
| CIP | 90 (58.8) | 54 (56.8) | 36 (62.1) |
| CN | 94 (61.4) | 51 (53.7) | 43 (74.1) |
| RIF | 71 (46.4) | 35 (36.8) | 36 (62.1) |
| LZD | 10 (6.5) | 4 (4.2) | 6 (10.3) |
| TEC | 38 (24.8) | 20 (21.1) | 18 (31) |
| AK | 70 (45.7) | 32 (33.7) | 36 (62.1) |
| MEM | 91 (59.4) | 51 (53.7) | 40 (69) |
| Total | 153 (100) | 63 (100) | 90 (100) |
4.3. Presence of IS256, icaABD and aap Genes
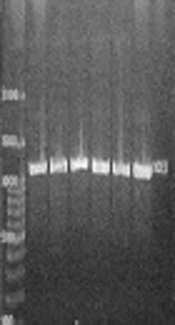
Genetic analysis of 153 S. epidermidis strains showed the presence of icaABD in 80 (52.2%) strains (Figure 2A), and the presence of aap gene in 136 (88.8%) isolates (Figure 2B), and the presence of IS256 gene in 139 (90%) isolates (Figure 2C). The pattern of antibiotic susceptibility was not different between the strains carrying the icaABD gene and those possessing the aap gene (P > 0.05). Additionally, among the strains with icaABD genes, only 53 (66.2%) exhibited the ability to form biofilms, and among the isolates with aap gene, 86 (63.2%) were biofilm positive yet there is no significant discrepancy in biofilm formation with the presence of icaABD and aap genes (P > 0.05). In addition, 27 (17.6%) and 50 (32.7) of non-biofilm producer strains carried the icaABD and aap genes, respectively.
Detection of the operon icaBD, and Aap and IS256 genes in S. epidermidis strains. (A) PCR results with primer for icaABD gene, (B) PCR results with primer for aap gene, (C) PCR results with primer for IS256 gene

4.4. Major Components of Zataria multiflora Extract
The compositional analysis of Zataria multiflora extract by GC/MS is shown in Table 3. The major components were phenol, 2-methyl-5-1-methylethyl (26.9%), Formamide, N-methoxy (21.7%), and thymol (16.4%).
Compositional Analysis of Zataria multiflora Extract
| Compounds | Percentage (%) | Retention Time |
|---|---|---|
| Propanoic acid,3-(acetylthio)-2-methyl | 4.51 | 5.569 |
| Formamide, N-methoxy | 21.7 | 5.923 |
| Dodecane | 4.13 | 16.546 |
| Thymol | 16.4 | 19.595 |
| Phenol,2-methyl-5-(1-methylethyl) | 26.9 | 19.87 |
| Tetradecane | 16 | 21.873 |
| Phenol,3,5-bis(1,1-dimethylethyl) | 0.72 | 24.954 |
| 7-methyl-Z-tetradecane-1-ol acetat | 7.62 | 26.643 |
| t- Buthyl-(1)-dimethyl-silane | 1.69 | 28.002 |
4.5. MIC and MBC of Zataria multiflora Essential Oil and Extract
To determine the MIC and MBC of Zataria multiflora, the biofilm forming isolates were selected and examined. The MBC and MIC of Zataria multiflora essential oil were 10% and sub-MIC concentration was 9%. Furthermore, the MBC and MIC of Zataria multiflora extract was 6 mg/mL and 5 mg/mL, respectively.
4.6. Phenotypic and Genotypic Impacts of Sub-Inhibitory Concentration (subMIC) of Essential Oil and Extract on Biofilm Formation
In order to determine the impact of Zataria multiflora extract and essential oil on biofilm forming ability and expression of genes involved in biofilm production in selected isolates, the sub-inhibitory concentration (subMIC) of essential oil and extract was tested. Among 109 biofilm positive isolates, the sub-MIC concentration of essential oil and extract reduced biofilm formation ability in 93 (85.3%) and 106 (97.2%), respectively.
In order to determine the effect of Zataria multiflora essential oil and extract on the expression of key genes involved in biofilm formation, the real time–PCR was used. The expression efficiency was between 97% and 103%. The results of sub-inhibitory concentration of essential oil and extract on the expression of icaA, icaD, and aap genes are shown in Figure 3. Based on the findings of Rest2009, the relative mRNA expression of icaA, icaD, and aap genes were reduced by 0.15, 0.1, and 0.07 folds compared to the negative control after treating the Staphylococcus epidermidis RP62A with subMIC concentration of essential oil (P < 0.001). In addition, the relative expression of icaA, icaD, and aap genes after exposure to the subMIC concentration of extract was lower (0.37, 0.42 and 0.41, respectively) than the negative control (P < 0.001).
The relative expression of icaA, icaD and aap genes after treating with sub-MIC concentration of essential oil (A) and extract (B)

5. Discussion
The present study investigated biofilm-forming strains of S. epidermidis by phenotypic and genotypic methods, and the association between biofilm production and antimicrobial resistance. The study used two methods including MtP for the evaluation of biofilm formation, and PCR for the detection of icaABD, IS256, and aap genes.
The findings were in agreement with Sahal and Bilkay (11) and Farajzadeh Sheikh and Mehdinejad studies (18) that showed the isolates of blood and wound were the most common clinical materials. The researchers demonstrated that in some isolates of S. epidermidis, sub-inhibitory concentrations of azithromycin, clarithromycin, and erythromycin enhanced biofilm formation in a dose-dependent manner (19). Based on Sahal and Bilkay study, S. epidermidis strains from blood, wound, and cardiothoracic surgery samples showed sensitivity to vancomycin, while 65% were resistant to all β-lactams and 60% were multi-drug resistant (11); researchers have suggested that vancomycin is an efficient antibiotic for inhibition of S. epidermidis (11, 20-22). The current results are in agreement with other studies that showed resistance to erythromycin (8, 19). Erythromycin is thought to be ineffective for the eradication of biofilms. In the current study on 153 strains, 109 (71.2%) isolates produced biofilm, and the current results showed no significant association between antibiotic resistance and biofilm formation.
Wojtyczka demonstrated that S. epidermidis showed high susceptibility to rifampicin, ciprofloxacin, and chloramphenicol yet less susceptibility to erythromycin and SXT trimethoprim/sulfamethoxazole. Wojtyczka evaluated 32 S. epidermidis strains and 12 (37.5%) were biofilm positive. There was no significant relationship between biofilm formation and antimicrobial resistance (8). In another study, out of 80 strains of S. epidermidis, 53 (66%) were biofilm positive and 12 (15%) were weak producers (23). It is believed that ica operon genes are associated with biofilm production (24). In this study, out of 135 strains, 53 (55.8%) possessed the icaBD gene yet did not exhibit biofilm formation. This result may be due to high frequency of biofilm producer isolates in the population. Eftekhar studied biofilm phenotype and ica operon genes carriage in two groups of isolates, clinical strains from symptomatic patients, and skin isolates from healthy patients. In total, 52% of the clinical strains and 56% of the skin isolates were biofilm-forming strains with ica operon genes carriage being 30% and 8%, respectively. Eftekhar indicated that aap is the most likely factor for biofilm formation via a PIA non-dependent pathway. In the current findings, the presence of the aap gene was 88.8% among 153 S. epidermidis isolates and showed no association with phenotype. Dadashi et al. reported that the chloroformic extract of Zataria multiflora had significant inhibitory impact on the ESBL-producing Klebsiella pneumoniae strains with MIC50 = 1.56 mg/mL and MIC90 = 3.12 mg/mL. Furthermore, methanolic and acetonic extracts showed lower impact with MIC50 of 3.12 and 6.25 mg/mL, respectively (6).
Of note, the presence of the aap gene was greater than IS256 and icaABD genes among blood samples. The current study revealed no association between the presences of IS256 as an invasiveness factor with clinical samples. Regarding MtP results, there was a greater number of isolates from wound and blood samples yet there was no significant association with icaABD and aap genes, therefore, the results demonstrate no particular pattern among the phenotypic with genotypic method.
Other studies have found no relationship between biofilm formation and the presence of the ica operon (25, 26). Analysis of data showed 27 (46.6%) strains with the icaABD gene and 50 (86.2%) strains with the aap gene among non-biofilm forming strains; these observations suggested there were other factors regulating biofilm formation (27, 28).
Comparing other studies and the current findings, it could be suggested that clinical and commensal isolates have similar virulence factors. In the current research, some strains were biofilm negative/ica positive and biofilm negative/Aap positive. In commensal strains, it may not be necessary to express ica operon genes and form biofilms, yet in clinical strains, in order to avoid the host immune system, expression of the ica operon or other factors is necessary. In conclusion, the expression of virulence factors depends on external environmental conditions, such as the presence of patient’s immune system. In the current findings, there were both biofilm positive/ica negative and biofilm positive/Aap negative strains, and that the environmental conditions determine which virulence factors S. epidermidis expresses to ensure survival.
The phenolic components of extracts derived from Zataria multiflora are predominant; however, geographic differences, age, and preparation protocol could alter the content of extracts. Carvacrol (61.29%) and thymol (25.18%) were the dominant components in Zataria multiflora extract from Yazd (3) yet in Fars plant, only carvacrol (71.12%) was seen in the extract content (4). The major content of the extract contains thymol and phenol compounds, however, there was no carvacrol compound in the extract. The presence of thymol and phenolic compound results in antimicrobial impacts. Based on the outcomes, the extracts of Zataria multiflora had anti-bacterial and anti-biofilm effects against S. epidermidis. There are a few findings concerning the impacts of Zataria multiflora on S. epidermidis. In the work by Mahboubi et al., the MIC of Zataria multiflora was 1.56 mg/mL (29), which is lower than the current findings (5 mg/mL). However, according to the researcher’s knowledge, there is no document to assess the effects of Zataria multiflora on biofilm forming phenotype and the expression of genes involved.
The current study is the first to report the anti-biofilm property of Zataria multiflora. This effect is both phenotypic and genotypic and leads to significant attenuation of mRNA expression of momentous genes involved in biofilm formation, such as icaA, icaD, and aap genes. Given the significant inhibitory effect of the extract and essential oil of Zataria multiflora on biofilm formation, it seems that these substances are good options for studies related to controlling biofilm formation in Staphylococcus epidermidis.
Acknowledgements
References
-
1.
Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;100(1):1-18. [PubMed ID: 18366134]. [PubMed Central ID: PMC2706312]. https://doi.org/10.1002/bit.21838.
-
2.
Kampf G, Loffler H, Gastmeier P. Hand hygiene for the prevention of nosocomial infections. Dtsch Arztebl Int. 2009;106(40):649-55. [PubMed ID: 19890431]. [PubMed Central ID: PMC2770229]. https://doi.org/10.3238/arztebl.2009.0649.
-
3.
Shafiee A, Javidnia K. Composition of essential oil of Zataria multiflora. Planta Med. 1997;63(4):371-2. [PubMed ID: 17252397]. https://doi.org/10.1055/s-2006-957707.
-
4.
Azizkhani M, Misaghi A, Basti AA, Gandomi H, Hosseini H. Effects of Zataria multiflora Boiss. essential oil on growth and gene expression of enterotoxins A, C and E in Staphylococcus aureus ATCC 29213. Int J Food Microbiol. 2013;163(2-3):159-65. [PubMed ID: 23558199]. https://doi.org/10.1016/j.ijfoodmicro.2013.02.020.
-
5.
Saito ST, Trentin Dda S, Macedo AJ, Pungartnik C, Gosmann G, Silveira Jde D, et al. Bioguided fractionation shows Cassia alata extract to inhibit Staphylococcus epidermidis and Pseudomonas aeruginosa growth and biofilm formation. Evid Based Complement Alternat Med. 2012;2012:867103. [PubMed ID: 22548121]. [PubMed Central ID: PMC3323858]. https://doi.org/10.1155/2012/867103.
-
6.
Dadashi M, Hashemi A, Eslami G, Fallah F, Goudarzi H, Erfanimanesh S, et al. Evaluation of antibacterial effects of Zataria multi fl ora Boiss extracts against ESBL-producing Klebsiella pneumoniae strains. Avicenna J Phytomed. 2016;6(3):336-43. [PubMed ID: 27462557]. [PubMed Central ID: PMC4930541].
-
7.
Bannerman TL, Peacock S, Murray P, Baron E, Jorgensen J, Landry M, et al. Staphylococcus, Micrococcus and other catalase-positive cocci. Manual Clin Microbiol. 2006:390-411.
-
8.
Wojtyczka RD, Orlewska K, Kepa M, Idzik D, Dziedzic A, Mularz T, et al. Biofilm formation and antimicrobial susceptibility of Staphylococcus epidermidis strains from a hospital environment. Int J Environ Res Public Health. 2014;11(5):4619-33. [PubMed ID: 24776724]. [PubMed Central ID: PMC4053877]. https://doi.org/10.3390/ijerph110504619.
-
9.
Merritt JH, Kadouri DE, O'Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2005;Chapter 1:Unit 1B 1. [PubMed ID: 18770545]. [PubMed Central ID: PMC4568995]. https://doi.org/10.1002/9780471729259.mc01b01s00.
-
10.
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996-1006. [PubMed ID: 3905855]. [PubMed Central ID: PMC271866].
-
11.
Sahal G, Bilkay IS. Multi drug resistance in strong biofilm forming clinical isolates of Staphylococcus epidermidis. Braz J Microbiol. 2014;45(2):539-44. [PubMed ID: 25242939]. [PubMed Central ID: PMC4166280].
-
12.
Kozitskaya S, Cho SH, Dietrich K, Marre R, Naber K, Ziebuhr W. The bacterial insertion sequence element IS256 occurs preferentially in nosocomial Staphylococcus epidermidis isolates: Association with biofilm formation and resistance to aminoglycosides. Infect Immun. 2004;72(2):1210-5. [PubMed ID: 14742578]. [PubMed Central ID: PMC321601].
-
13.
Djalalinia S, Owlia P, Forouzan AS, Habibi E, Dejman M, Eftekhari MB, et al. Health research evaluation and its role on knowledge production. Iran J Public Health. 2012;41(2):39-46. [PubMed ID: 23113133]. [PubMed Central ID: PMC3481679].
-
14.
Lou Q, Qi Y, Ma Y, Qu D. Two-component signal transduction system SaeRS positively regulates Staphylococcus epidermidis glucose metabolism. ScientificWorldJournal. 2014;2014:908121. [PubMed ID: 24592198]. [PubMed Central ID: PMC3921950]. https://doi.org/10.1155/2014/908121.
-
15.
Tormo MA, Marti M, Valle J, Manna AC, Cheung AL, Lasa I, et al. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J Bacteriol. 2005;187(7):2348-56. [PubMed ID: 15774878]. [PubMed Central ID: PMC1065223]. https://doi.org/10.1128/JB.187.7.2348-2356.2005.
-
16.
Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39(6):2151-6. [PubMed ID: 11376050]. [PubMed Central ID: PMC88104]. https://doi.org/10.1128/JCM.39.6.2151-2156.2001.
-
17.
Juarez-Verdayes MA, Ramon-Perez ML, Flores-Paez LA, Camarillo-Marquez O, Zenteno JC, Jan-Roblero J, et al. Staphylococcus epidermidis with the icaA(-)/icaD(-)/IS256(-) genotype and protein or protein/extracellular-DNA biofilm is frequent in ocular infections. J Med Microbiol. 2013;62(Pt 10):1579-87. [PubMed ID: 23861297]. https://doi.org/10.1099/jmm.0.055210-0.
-
18.
Farajzadeh Sheikh A, Mehdinejad M. Identification and determination of coagulase-negative Staphylococci species and antimicrobial susceptibility pattern of isolates from clinical specimens. Afr J Microbiol Res. 2012;6(8). https://doi.org/10.5897/ajmr11.076.
-
19.
Wang Q, Sun FJ, Liu Y, Xiong LR, Xie LL, Xia PY. Enhancement of biofilm formation by subinhibitory concentrations of macrolides in icaADBC-positive and -negative clinical isolates of Staphylococcus epidermidis. Antimicrob Agents Chemother. 2010;54(6):2707-11. [PubMed ID: 20231401]. [PubMed Central ID: PMC2876384]. https://doi.org/10.1128/AAC.01565-09.
-
20.
Doğruman Al F AG, Sipahi B, Sultan N. Kan örneklerinden soyutlanan stafilokok suşlarının antibiyotiklere direnç durumları. Ankem Derg. 2005;19(1):14-6.
-
21.
Chaieb K, Abbassi MS, Touati A, Hassen AB, Mahdouani K, Bakhrouf A. Molecular characterization of Staphylococcus epidermidis isolated from biomaterials in a dialysis service. Annal Microbiol. 2005;55(4):307.
-
22.
McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: Pathogenesis and clinical management. J Pharm Pharmacol. 2008;60(12):1551-71. [PubMed ID: 19000360]. https://doi.org/10.1211/jpp/60.12.0001.
-
23.
Arciola CR, Campoccia D, Gamberini S, Donati ME, Pirini V, Visai L, et al. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials. 2005;26(33):6530-5. [PubMed ID: 15949842]. https://doi.org/10.1016/j.biomaterials.2005.04.031.
-
24.
Fredheim EG, Klingenberg C, Rohde H, Frankenberger S, Gaustad P, Flaegstad T, et al. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol. 2009;47(4):1172-80. [PubMed ID: 19144798]. [PubMed Central ID: PMC2668337]. https://doi.org/10.1128/JCM.01891-08.
-
25.
Ninin E, Caroff N, Espaze E, Maraillac J, Lepelletier D, Milpied N, et al. Assessment of ica operon carriage and biofilm production in Staphylococcus epidermidis isolates causing bacteraemia in bone marrow transplant recipients. Clin Microbiol Infect. 2006;12(5):446-52. [PubMed ID: 16643521]. https://doi.org/10.1111/j.1469-0691.2006.01382.x.
-
26.
de Silva GD, Kantzanou M, Justice A, Massey RC, Wilkinson AR, Day NP, et al. The ica operon and biofilm production in coagulase-negative Staphylococci associated with carriage and disease in a neonatal intensive care unit. J Clin Microbiol. 2002;40(2):382-8. [PubMed ID: 11825946]. [PubMed Central ID: PMC153361].
-
27.
Eftekhar F, Mirmohamadi Z. Evaluation of biofilm production by Staphylococcus epidermidis isolates from nosocomial infections and skin of healthy volunteers. Int J Med Sci. 2009;1(10):438-41.
-
28.
Dobinsky S, Kiel K, Rohde H, Bartscht K, Knobloch JK, Horstkotte MA, et al. Glucose-related dissociation between icaADBC transcription and biofilm expression by Staphylococcus epidermidis: Evidence for an additional factor required for polysaccharide intercellular adhesin synthesis. J Bacteriol. 2003;185(9):2879-86. [PubMed ID: 12700267]. [PubMed Central ID: PMC154395].
-
29.
Mahboubi A, Kamalinejad M, Ayatollahi AM, Babaeian M. Total phenolic content and antibacterial activity of five plants of labiatae against four foodborne and some other bacteria. Iran J Pharm Res. 2014;13(2):559-66. [PubMed ID: 25237351]. [PubMed Central ID: PMC4157031].