Molecular barcoding confirms the presence of exotic Asian seaweeds (Pachymeniopsis gargiuli and Grateloupia turuturu) in the Cantabrian Sea, Bay of Biscay
- Published
- Accepted
- Received
- Academic Editor
- Bill Hooker
- Subject Areas
- Biodiversity, Ecology, Genetics, Marine Biology, Molecular Biology
- Keywords
- Exotics, rbcl, COI, Seaweeds, Bay of Biscay, Rhodophyta, Introduced species, Grateloupia, Halymeniaceae, DNA barcoding
- Copyright
- © 2017 Montes et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Molecular barcoding confirms the presence of exotic Asian seaweeds (Pachymeniopsis gargiuli and Grateloupia turuturu) in the Cantabrian Sea, Bay of Biscay. PeerJ 5:e3116 https://doi.org/10.7717/peerj.3116
Abstract
Background
The introduction of exotic species can have serious consequences for marine ecosystems. On the shores of the Cantabrian Sea (North of Spain) there are no routine examinations of seaweeds that combine molecular and morphological methods for early detection of exotic species making it difficult to assess in the early stages their establishment and expansion processes as a result of anthropogenic activities (e.g., shipping and/or aquaculture).
Methods
In this work we used both morphological identification and molecular barcoding (COI-5P and rbcL genes) of red algae collected in Asturias, Bay of Biscay (Gijón and Candás harbours) and from the University of Oviedo’s herbarium samples.
Results
The results confirmed the presence of exotic Asian seaweeds Pachymeniopsis gargiuli and Grateloupia turuturu Yamada on Cantabrian Sea shores. Several individuals of these species were fertile and developing cystocarps when collected, underlining the risk of possible expansion or continued establishment. This study constitutes the first report of the Asian P. gargiuli in this area of the Bay of Biscay.
Conclusions
Here the presence of the exotic species of the Halymeniales P. gargiuli is confirmed. We hypothesize that this species may have been established some time ago as a cryptic introduction with G. turuturu in Galician shores. The detection of these species on the shores of the Cantabrian Sea is relevant since introductions of Pachymeniopsis species could have been overlooked on other European coasts, probably mixed with G. turuturu and P. lanceolata. Our results confirm one new alien seaweed species that has been detected using molecular methods (COI-5P region and rbcL genes barcoding) on North Atlantic shores: the Asian native P. gargiuli. This demonstrates that routine screening for early detection of exotic algae in the Cantabrian Sea can be used for risk assessment. Genetic barcoding should be done using both rbcL gene and COI-5P regions since, although COI-databases are still poorer in sequences and this inhibits successful outcomes in Grateloupia-related species identifications, it is nonetheless a useful marker for species-level identifications in seaweeds.
Introduction
The problem of invasion is considered one of the main threats to global biodiversity (Nunes et al., 2014). Seaweed species introductions are a significant component of Non-Indigenous Marine Species (NIMS) introductions. When these become invasive they can rapidly spread and monopolize space, alter food webs, and modify both ecosystem structure and function (Thresher, 2000; Katsanevakis et al., 2014). Shipping has been reported as the most important pathway for the introduction of NIMS and in recent years more than a thousand marine alien species have been reported in European seas, even though efforts to monitor and report alien species vary among European countries (Katsanevakis et al., 2013).
Grateloupia C. Agardh is the largest, least characterized and most taxonomically fluctuating genus of the family Halymeniaceae, with many poorly characterized species that display a wide array of morphological traits ranging from pinnate to subdichotomous to foliose morphologies (Guiry & Guiry, 2015; Kim et al., 2014). Recent studies (Gargiulo, Morabito & Manghisi, 2013) have resulted in a continued taxonomical rearrangement of this genus, including reinstatement of the genera Pachymeniopsis Y. Yamada ex S. Kawabata (Kawaguchi, 1997), Prionitis J. Agardh (Wang et al., 2001), Dermocorynus H. Crouan et P. Crouan (Wilkes, Mcivor & Guiry, 2005) and Phyllymenia J. Agardh (De Clerck et al., 2005b). Some Grateloupia species have also been shown to be highly invasive via marine transportation and/or aquaculture activities (e.g., Gavio & Fredericq, 2002; Nyberg & Wallentinus, 2005; Verlaque et al., 2005; Verlaque, Boudouresque & Mineur, 2007; Hewitt, Campbell & Schaffelke, 2007; Saunders & Withall, 2006; Mathieson et al., 2008; Miller, Hughey & Gabrielson, 2009; DePriest & Lopez-Bautista, 2012). Grateloupia is represented in the North European Atlantic by more than six species (Gavio & Fredericq, 2002; Wilkes, Mcivor & Guiry, 2005) of which only two, G. filicina (J.V. Lamouroux) C. Agardh, and G. turuturu Yamada, are considered introduced (De Clerck et al., 2005a; De Clerck et al., 2005b).
Morphological analysis alone can be ineffective for species identification and leave cryptic introductions undetected (e.g., Saunders, 2009). Molecular methods have been proven to be more effective, but morphological analysis is still the most frequent (and sometimes the only) method used for seaweed NIMS routine screenings in northern Spain (e.g., Bárbara & Cremades, 2004; Cires & Moliner, 2010). Recently four non-foliose Grateloupia-like samples from Gijón marina and University of Oviedo FCO Herbarium samples were identified as G. imbricata Holmes and the Mediterranean G. filicina (J.V. Lamuroux) C Agardh, two Halymeniales exotic in that area (Montes et al., 2016). This demonstrated the potential for molecular methods to detect previously overlooked species introductions and in particular, other possible introduction events for Grateloupia and other Halymeniales similar to the ones found on Galician coasts (G. doryphora and G. turuturu), in the coasts of the Bay of Biscay.
Grateloupia turuturu, a species considered highly invasive on a global scale (Nyberg & Wallentinus, 2005 as G. doryphora [Montagne] M.Howe) is noted as being among the invasive Grateloupia species on the northern coast of Spain, in Galicia (Bárbara et al., 2005; Boletín Oficial Del Estado, España, 2013). Grateloupia turuturu was discovered in samples considered to be G. doryphora that were screened using molecular identification tools (Bárbara & Cremades, 2004). Curiously, the most recent checklist for benthic algae in Asturias (Cires & Moliner, 2010) does not cite records of G. turuturu on Asturian coasts but it does mention the high probability of an undetected establishment. Since some confusion exists in Grateloupia species identification (e.g., Gavio & Fredericq, 2002; Kim et al., 2013; Montes et al., 2016), and since there are no routine NIMS screenings combining both molecular and anatomical methods on the coasts of the Cantabrian Sea, it is likely that a foliose Grateloupia species similar to the G. doryphora group of poorly identified species (e.g., G. turuturu) could be expanding via anthropogenic activities (i.e., shipping and aquaculture) as been demonstrated for many other marine species in this region (Arias et al., 2014a; Arias et al., 2014b; Habtemariam et al., 2015; Semeraro et al., 2016).
This work describes a routine screening of foliose, putatively identified Grateloupia specimens using morphological identification as well as COI-5P and rbcL genes as barcodes. Both genes were proven to be effective for species identification in red algae after blasting new sequences against BOLD (Barcode of Life Database) and GenBank databases (e.g., Saunders, 2005; Saunders & McDevit, 2012). The aim of this study was to evaluate the status of possible exotic Halymeniales introductions to the Cantabrian Sea (as outlined in Cires & Moliner, 2010).
Materials and Methods
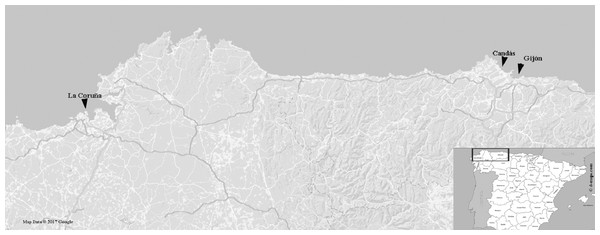
In this work, a barcoding routine screening of foliose Grateloupia seaweeds was carried out in the large commercial port of Gijón (González, 2012 and references therein) and Candás harbour, a nearby smaller fishing and recreational harbour. Seaweed specimens were collected from jetties in the sport wharf of the inner part of the harbour of Gijón (43°32′43″N–5°39′44″W) and from the main wharf of Candás (43°58′79″N–5°75′85″W) during a low tide (Fig. 1). Samples collected were foliose Halymeniales (24 samples) preliminarily identified as Grateloupia turuturu on the basis of external morphology: long foliose fronds and pseudo-dichotomously branched blades of a dark reddish brown colour and mucilaginous-but-firm texture. Samples were air-dried and stored at −4 °C in the FCO Herbarium of the University of Oviedo (http://www.unioviedo.es/bos/Herbario/FCO.htm) (Table 1).
Figure 1: Map showing the sampling regions.
La Coruña, Galicia (43°48′05″N–6°47′16″W), where FCO 1583 and FCO 1584 were collected; Candás, Asturias (43°58′79″N–5°75′85″W), where FCO 2076, FCO 2077, FCO 2135, FCO 2136, (FCO 2138–FCO 2140) were collected; and Gijón, Asturias (43°32′43″N–5°39′44″W) where FCO 2134, (FCO 2141–FCO 2143) were collected. Map data © 2017 Google Instituto de Geografía Nacional, Spain map taken from URL: http://d-maps.com/carte.php?num_car=5674&lang=es.Samples already deposited at FCO were also used as external controls to this study and for species confirmation using genetic tools. The samples with voucher codes FCO 1583 and FCO 1584 were collected in 2001 from La Coruña (43°48′05″N–6°47′16″W) (Fig. 1) and classified, through classical taxonomy, as G. doryphora. In addition, we included the samples with the voucher codes FCO 2076 and FCO 2077, collected in 2010 from Candás (43°58′79″N–5°75′85″W) with previous morphological classifications as G. turuturu. Old voucher samples were rehydrated during one day in seawater prior to morphological analysis.
| Location | Collector/ Date | FCO Numbers | COI-5P | rbcL | |||||
|---|---|---|---|---|---|---|---|---|---|
| GenBank Number | Results of Assignments in BOLD System | Results of Assignments in GeneBank database. | GenBank Number | Results of Assignments in BOLD System | Results of Assignments in GeneBank database. | ||||
| ASTURIAS | GIJÓN 43°32′43″N–5°39′44″W | JM Rico M Montes 4∕4∕2014 | FCO 2134, (FCO 2141-FCO 2143) | KP271163 | Prionitis sp. 3jeju ABMMC 11722-10 (99.7%) | Grateloupia sp. KJ648553.1 (99.9%) | KP281326 | Grateloupia sp. AY651060.1 (100%) | Grateloupia sp. AY651060.1 (100%) |
| CANDÁS 43°58′79″N - 5°75′85″W | JM Rico M Montes 4∕21∕2014 | FCO 2135, FCO 2136, (FCO 2138–FCO 2140) | |||||||
| J Raboso. 5∕6∕2010 | FCO 2076 | ||||||||
| J Raboso 5∕25∕2010 | FCO 2077 | ||||||||
| GALICIA | LA CORUÑA 43°48′05″N–6°47′16″W | JM Rico 9∕18∕2001 | FCO 1584 | ||||||
| FCO 1583 | KP271166 | Grateloupia turuturu ABMMC 1360-07 (100%) | Grateloupia turuturuKF475725.1 (100%) | KP281329 | Grateloupia turuturuGU168561.1 (99,9%) | Grateloupia turuturuAB809603.1 (99,9%) | |||
Freezing microtome sections and staining were carried out on all samples following Rico & Guiry (1997) using a cryotome (Leica, Germany, Model CM1510-1, Fabr. No. 2303/07.2000, Cat. No. 043631515) and blue aniline for staining, to conduct morphological analyses. DNA was extracted using the GeneMATRIX Plant and Fungi DNA purification Kit (EURx Cat. No. E3595, Roboklon GmbH, Berlin, Germany; GeneMATRIX purification Kit) using 20–70 mg of each sample for both FCO Herbarium and the fresh samples obtained at Candás and Gijón. Plant material was ground in a mortar using liquid nitrogen until pulverized material for DNA extraction was obtained. The extracted DNA was stored at −20 °C. PCRs were performed for both rbcL gene and COI-5P regions. Following Freshwater & Rueness (1994) and Gavio & Fredericq (2002), three different combinations of primers (F7-R753, F577-R1381 and F993-RrbcS) were used to obtain three overlapping fragments of the rbcL gene. The primer pair GazF1 (Saunders, 2005) and GazR4 (Saunders, 2008) were used for COI amplification. PCRs used these general conditions: 3 mM MgCl2, 1x of PCR Buffer, 0.4 mM dNTPs, 0.3 µM from both primers and 1u of Taq Polymerase, all in a 20 µl volume (including 2 µl of DNA extracts). PCR amplification profiles were 95 °C for 5 min; 5 cycles of 95 °C for 30 s, 42 °C annealing for 1 min, 72 °C extension for 1 min; followed by 35 cycles of 95 °C for 30 s, 46.5 °C annealing for 1 min, 72 °C extension for 1 min followed by 72 °C final extension for 10 min for COI-5P; and 95 °C for 5 min; 40 cycles of 95 °C for 1 min, 42 °C annealing for 1 min, 72 °C extension for 1 min 30 sec; followed by 72 °C final extension for 10 min for rbcL. These profiles are similar to those from Saunders & Moore (2013). The PCR products were electrophoresed in a 2% agarose gel, containing SimplySafe™ (EURx Cat. No. E4600-01) and using Promega 100 bp DNA Ladder Molecular Weight Marker (Promega Corporation 2800 Woods Hollow Road Madison, WI 53711, USA) for band sizes inspections. Bands were cut from agarose gels and were purified using the standard protocol of the EURx agarose purification kit (EURx Cat. No. E3540-02, Pryzodnikow, Gdansk, Poland) and sent to MACROGEN (Amsterdam, Netherlands) for sequencing using the standard Sanger sequencing method (Sanger & Coulson, 1975).
The new sequences were manually checked and edited using the freeware BIOEDIT (Hall, 1999). Alignments were made using CLUSTALW (Thompson, Higgins & Gibson, 1994). The different sequences found in this study were submitted to GenBank. After alignment and corrections, species identification was carried out using Blast to search BOLD and GenBank databases. Species identifications were accepted if they showed more than 98% similarity to the reference sequences available in both databases. Additional Grateloupia sequences obtained from GenBank were used in downstream phylogenetic analyses, and Halymenia floresii (Clemente) C. Agardh (KJ594956 and GQ862071) was used as an outgroup (Table S1). Cluster analysis was performed using the Neighbor-joining method in MEGA v6 software (Tamura et al., 2013), the Tamura-Nei DNA evolution model with invariable sites (TN93 + I) for COI-5P, and Tamura 3-Parameter with gamma distribution evolution model (T92 + G) for rbcL. Sequences were trimmed to 615 bp (located on the 216–831 bp region in the 3′ end) in order to include database derived sequences of varying lengths that were identified as the most likely DNA models (ModelTest software available inside MEGA v6). A total of 2,000 bootstrap steps were conducted for testing branch supports.
This study was approved by the Committee of Ethics of the Principado de Asturias, with the reference 100/06 for GRUPIN-2014-093.
Results
Morphology
Samples FCO 2077, FCO 2134, FCO 2140, FCO 2143
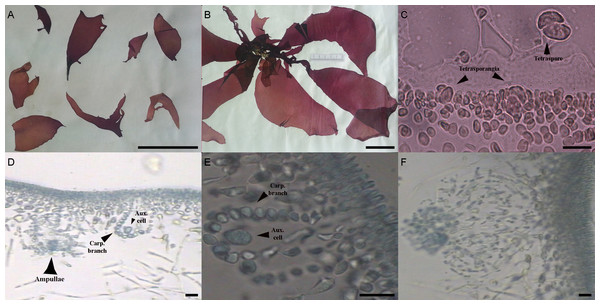
The morphological characters of the University of Oviedo herbarium (FCO 2077) and freshly obtained samples (FCO 2134, FCO 2140, FCO 2143) were clearly those of the genus Grateloupia (Fig. 2) as they presented a habit typical of this genus, with specimens ranging from 4 to 30 cm long, with a firm texture, colors ranging from purplish-red to reddish brown and large fronds with lanceolate to linear, pseudo-dichotomously branched blades. The blades were membranaceous, lubricous and pseudo-dichotomously branched, branching from the discoid holdfast (Figs. 2A and 2B). Multiaxial section of internal structures showed a narrow cortical zone and a broad filamentous medullary zone; the cortical zone was composed of 6 layers of cells, 3 cylindrical-roundish cells of 5–10 µm long and 2 µm wide; medulla consisting of loose medullary anticlinally arranged filaments; inner cortex composed of 2–3 roundish cells of 5–10 µm diameter and extracellular mucilaginous material (Figs. 2A and 2B). The specimens found in both harbours during this study were fertile. Carpogonial branch 6-celled ampullar structure and post-fertilization events were in agreement with those in the type of the genus (Figs. 2D and 2E; Gargiulo, Morabito & Manghisi, 2013). Auxiliary cell ampullae consisted of an oval auxiliary cell, and 2–3 unbranched ampullary filaments, up to 10-cells long (Figs. 2D and 2E), similar to illustrations shown in Wilkes, Morabito & Gargiulo (2006). Tetrasporangia were detected in herbarium sample FCO 2077 and were isomorphic, arising from inner cortical cells, cruciately divided, embedded beneath the cortical surface and 25–30 µm in diameter (Fig. 2C). Cystocarps were similar to others reported both for G. lanceolata (Okamura) Kawaguchi (Gargiulo, Morabito & Manghisi, 2013) and to the FCO 2137 sample (Montes et al., 2016) with a diameter around 120 µm (Fig. 2F).
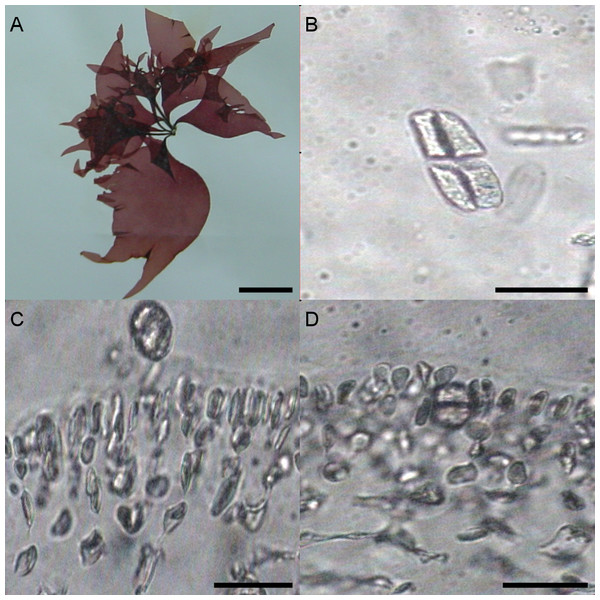
University of Oviedo herbarium sample FCO 1583
Different morphological characteristics were found for the University of Oviedo herbarium sample FCO 1583. Fronds were 15 cm long and thinner than all the other samples (Fig. 3A). The transverse section of the middle of the frond showed a narrow cortical zone and a broad filamentous medullary zone; the cortex was formed by a 5–6 cell layer, with a conspicuous transition to the medulla (Figs. 3C, 3D). The outer cortical cells were roundish or cylindrical and 8–4 µm in diameter; medulla consisted of loose anticlinal arranged filaments. Tetrasporangia were isomorphic, arising from inner cortical cells as well but with slightly different morphology and 20–25 µm long and 5–20 µm wide (Figs. 3B–3D).
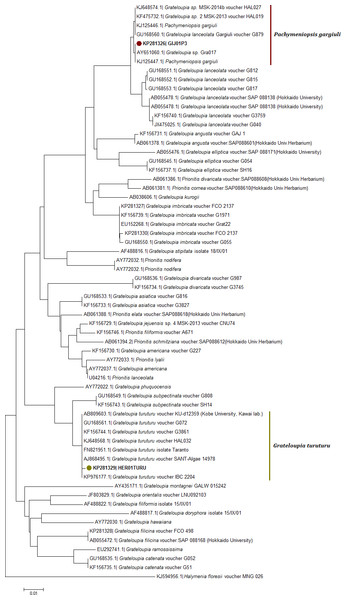
Figure 4: Neighbor joining consensus trees using partial sequences of COI gene.
Neighbor joining consensus trees using partial sequences (530 bp) of COI gene and the DNA evolution model TN93 + I. Nodes including samples from this study appear in color.Genetics
Four sequences, two for each gene under analysis (rbcL and COI-5P), were obtained in this work. The BLAST and NJ tree analyses demonstrated that these sequences belong to two different species Pachymeniopsis gargiuli S.Y. Kim, A. Manghisi, M. Moribato & S.M. Boo (Kim et al., 2014) and Grateloupia turuturu.
Pachymeniopsis gargiuli
Genetic analysis of the COI-5P gene from the samples collected from Gijón and Candás (Table 1), as well as from herbarium samples FCO 2076, FCO 2077 and FCO 1584 revealed a unique COI haplotype (KP271163) of 530 bp for all of them. Analysis of the rbcL gene in these samples revealed also only one haplotype (KP281326) of 1,190 bp (Table 1).
Blast results for the COI KP271163 haplotype revealed unspecific genetic identification matching with an unidentified Halymeniales species found in Korea labelled as ‘Prionitis sp. 3jeju’ (99.7% similarity) in the BOLD database and with Grateloupia sp. voucher GT103 (KJ648553; 99.9% similarity) in GenBank; both vouchers came from samples collected in Asia.
Blast of the rbcL KP281326 haplotype against the GenBank database did not support precise genetic identification. It matched only Grateloupia sp. Gra017 (100% similarity), an unspecified type of Grateloupia found in the Straits of Messina, Italy (Wilkes, Morabito & Gargiulo, 2006).
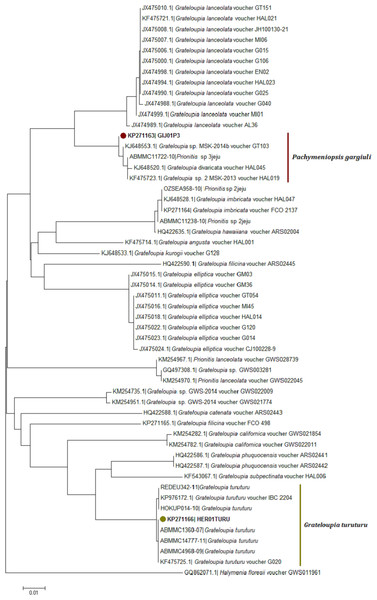
Neighbour-joining trees were generated using all available COI-5P sequences from the two databases (Fig. 4; Table S1). The COI KP271163 haplotype was located within a very well supported branch (clade) with Prionitis sp. 3jeju and G. divaricata Okamura and an unidentified Grateloupia species labelled as G. sp. MSK 2013-14 (KJ648553, KF475723) from Korea. All these sequences formed a monophyletic clade together, apart from the highly related G. lanceolata (which has been synonymised with Pachymeniopsis lanceolata (K. Okamura) Y. Yamada ex S. Kawabata (Kim et al., 2014) (Fig. 4).
The rbcL NJ tree (Fig. 5) revealed for the haplotype KP281326 a localization within a clade with KJ648574 (G. sp. MSK 2013) and with a species labelled as G. lanceolata gargiuli (GU168560) (Fig. 5). This clade has been recently identified as a gene pool grouping for the new species Pachymeniopsis gargiuli S.Y. Kim, A. Manghisi, M. Moribato & S.M. Boo (Fig. 5). This clade is clearly an independent entity in regard to P. lanceolata sequences (Fig. 5).
Figure 5: Neighbor joining consensus trees using partial sequences of rbcL gene.
Neighbor joining consensus trees using partial sequences (615 bp, located on the 216 to 831 bp region in the 3′ end) of rbcL gene and the DNA evolution model T92 + G. Nodes including samples from this study appear in color.Grateloupia turuturu
Genetic analysis of the Herbarium sample FCO 1583 produced sequences for each of the genes under study, KP271166 for COI-5P and KP281329 for rbcL (Table 1). Blast for both genes gave a precise and specific identification. The COI KP271166 sequence showed significant similarity with G. turuturu using both the BOLD database and GenBank (G. turuturu, KF475725) (Table 1). The KP281329 sequence was also identified (99.9% similarity) as G. turuturu in both databases (Table 1).
The COI-5P NJ tree revealed that the sequence KP271166 was part of a well-supported monophyletic clade with other G. turuturu sequences from Asian areas (Fig. 4; Table S1). The rbcL NJ tree showed the sequence KP281329 forming a monophyletic clade with G. turuturu samples from various places including the native Asian areas (KJ648568 and GU168561) (Fig. 5).
Discussion
Morphological analysis was not sufficient for precise species identification in this species complex. Despite this, we were able to determine the genus of some of the samples collected; the vegetative structure was typical of Grateloupia-type genera, and the reproductive structures analysis was similar to that used to separate between Grateloupia and Pachymeniopsis (Gargiulo, Morabito & Manghisi, 2013). Unfortunately not all the samples showed reproductive structures. Molecular analysis was by far the most effective method of species livel identification in this work and rbcL sequences supported much more precise identifications than COI sequences as previously reported for G. imbricata and G. filicina in Montes et al. (2016). This was an expected outcome given that the systematics and taxonomy of the Grateloupia spp. complex and related genera (e.g., Pachymeniopsis) have been proposed, established, discussed and rearranged using the rbcL gene (i.e., Wang et al., 2001; Wilkes, Mcivor & Guiry, 2005; De Clerck et al., 2005a; De Clerck et al., 2005b; Lin, Liang & Hommersand, 2008; Lee et al., 2009; Zhang et al., 2012; Gargiulo, Morabito & Manghisi, 2013; Yang & Kim, 2015). However, the COI gene has potential to become an equally effective marker for species identifications in red algae in the future as outlined in the past in other genera (Saunders, 2005; Freshwater et al., 2010; Saunders & Moore, 2013) supported by our (limited) success here. More data in COI genetic databases would help to overcome this obstacle. Moreover, in the COI-5P tree (Fig. 4) the KP271163 sequence was grouped in a complex clade including incomplete labelled species (e.g., Grateloupia sp. 2 MSK-2013 voucher HAL019) and one species with a correct taxonomic label, G. divaricata Okamura (Wang et al., 2001), a species that shows pinnated frond morphology (different from our samples), suggesting a species misidentification. This pointed to several errors in species identifications that appear in genetic databases. Fortunately, the rbcL tree (Fig. 5) clearly showed the correct identity since samples from this study showed 100% identity with sequences of P. gargiuli (KJ125446, KJ214447), a recently described species closely related to P. lanceolata as described by Kim et al. (2014).
Herbarium samples FCO 1583 and FCO 1584 were collected in the same area, the same day, and shared similar morphology; this led to both being identified as G. doryphora in 2001. The herbarium sample FCO 1583 has been unambiguously identified here as G. turuturu. However, the FCO 1584 sample was identified as P. gargiuli. Both samples were thus registered with incorrect species names in the FCO herbarium. G. turuturu presented a similar habit (Fig. 3A) to P. gargiuli (Figs. 2A and 2B) and vegetative and reproductive morphology was not sufficient for species identification. This underscores the value of genetic analyses if the aim is precise red algae species identifications when cryptic species complexes are considered with reproductive morphological features that are difficult to find if for example, samples do not include fertile specimens. Moreover, our results support the idea that both of the species we identified have been present in the Cantabrian Sea at least since 2001, although only G. turuturu has for the time being been described on Galician coasts (Bárbara et al., 2005). This last observation suggests that the presence of P. gargiuli in Asturias could be the result of a more recent introduction event (at least, since 2010) in comparison with other exotic seaweeds.
Cires & Moliner (2010) suggested that G. turuturu could be present in Asturian shores as a consequence of its proximity to coasts where it has been detected (Galicia) and because this seaweed has been reported showing invasive spread in nearby areas such as Portugal and France (Simon, Gall & Deslandes, 2001; Araújo et al., 2011), which makes expansion to other areas more probable. Although samples initially labelled by us as G. turuturu were collected in Asturias (including voucher samples from 2010 collected in Candás), they were found to all be P. gargiuli. These misidentifications are not surprising since P. gargiuli was also initially misidentified as G. doryphora and G. turuturu until genetic analyses were carried out on samples from the Strait of Messina (AY651060) (Wilkes, Morabito & Gargiulo, 2006). This suggests that the introduction of P. gargiuli in the Cantabrian Sea shores could be a cryptic introduction, thanks to its morphological similarity to G. turuturu and also to the similarity of G. turuturu in habit to the Galician native G. lanceola J. Agardh (J. Agardh). The latter similarity has resulted in a tendency to overlook the extent of G. turuturu presence in Galicia in the first place (Bárbara & Cremades, 2004).
P. gargiuli is also considered cryptic for P. lanceolata, sharing many morphological characteristics as well as distribution area in Korea (Kim et al., 2014). Samples of this species were detected in Italy (Wilkes, Morabito & Gargiulo, 2006) and, in the Canary Islands, initially identified as P. lanceolata (EU024819) (García-Jiménez et al., 2008), and also in the Madeira Islands at least since 2006; P. lanceolata was described, but only via morphological analysis (Ferreira et al., 2012). The similarity between these seaweeds raises the clear possibility of the presence of P. gargiuli in Madeira. The red algae G. imbricata was also found in Canarias through genetic analysis and in Madeira through classical taxonomy (García-Jiménez et al., 2008; Ferreira et al., 2012), but it has to date not been described anywhere in North Atlantic European shores or marinas except in the Gijón area (Montes et al., 2016). These similarities in locations where it was detected as an introduced species may suggest that these algae shared the same or similar introduction vectors/routes. The most likely and accepted hypothesis regarding introduction vectors of Pachymeniopsis spp. and of G. turuturu is oyster commerce to the Thau lagoon from the Pacific (Verlaque et al., 2005; Verlaque, Boudouresque & Mineur, 2007). It is likely that shipping will play a pivotal role in the range expansion of these species to the continent through ballast water and hull fouling (Hewitt, Campbell & Schaffelke, 2007).
This is the first report of a new genus, Pachymeniopsis, on the European shores of the North Atlantic. P. gargiuli is a species native to Asia, which was probably introduced long ago (at least since 2001) as a cryptic introduction within G. turuturu to Galician shores. Several individuals of these species were fertile as they were developing cystocarps and tetraspores when collected, emphasizing the risk of expansion or continued establishment. The detection of these species on the coasts of the Cantabrian Sea also indicates that Pachymeniopsis introductions may have been overlooked along other European coasts, especially if intermingled with G. turuturu and P. lanceolata. Our results highlight the potential for exotic algal introductions being missed when morphological identification fails to differentiate between highly similar species, and thus the importance of routine molecular barcoding surveys. This study also highlights the existence of gaps in the COI-5P records for Grateloupia spp., which might necessitate using an alternative barcode in the form of rbcL. We confirm previous findings and reports on two previously overlooked exotic algal introductions to an area of Europe where these had not been detected by morphology alone, the Asian native G. imbricata and the Mediterranean native G. filicina (De Clerck et al., 2005a; De Clerck et al., 2005b), and we add a newly reported species (Montes et al., 2016), also utilizing DNA barcoding, using both COI-5P region and rbcL gene: the exotic P. gargiuli. This demonstrates the utility of routine screenings that combine both anatomical investigation and barcoding procedures for early detection of exotic algae in the Cantabrian Sea. For such studies it would be ideal to combine anatomical and DNA barcoding procedures. In our case morphological examination was able to determine accurate genus placement, but for species identification, genetic methods proved to be more effective than conventional morphological identification.